Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
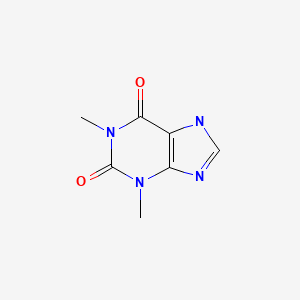
CASRN: 58-55-9
Drug Levels and Effects
Summary of Use during Lactation
Expert opinion considers use of theophylline to be acceptable during breastfeeding.[1,2] Maternal theophylline use may occasionally cause stimulation and irritability and fretful sleep in infants. Newborn and especially preterm infants are most likely to be affected because of their slow elimination and low serum protein binding of theophylline. There is no need to avoid theophylline products; however, keep maternal serum concentrations in the lower part of the therapeutic range and monitor the infant for signs of theophylline side effects. Infant serum theophylline concentrations can help to determine if signs of agitation are due to theophylline. Avoiding breastfeeding for 2 hours after intravenous or 4 hours after an immediate-release oral theophylline product can decrease the dose received by the breastfed infant. When theophylline is given as an oral sustained-release product, timing of nursing with respect to the dose is of little or no benefit.
Drug Levels
Maternal Levels. Theophylline rapidly equilibrates between plasma and milk. Peak milk levels occur 1 to 3 hours after oral ingestion of immediate-release products and almost immediately after intravenous administration. Milk levels parallel serum levels closely and average about 70% of simultaneous maternal serum levels.[3,4] Assuming that each 1 mg/kg of maternal theophylline increases her serum level by 2 mg/L, an exclusively breastfed infant would receive about 21% of the maternal weight-adjusted dosage of theophylline or 17% of the maternal dosage of aminophylline.
A physiologically based pharmacokinetic model was created for theophylline that predicted an average relative infant dose of 13% and a maximum of 17%.[5]
Infant Levels. Theophylline is found in the serum of breastfed infants.[6] In newborn infants with typical theophylline clearance rates, infant serum levels are expected to be between 1 and 4 mg/L with a maternal serum level in the therapeutic range of 10 to 20 mg/L.[3] Infant serum levels might occasionally accumulate to therapeutic levels in infants with slow clearance rates of the drug.[7]
A physiologically based pharmacokinetic model was created for theophylline that predicted an average serum concentration in a preterm neonate was 6.8 mg/L.[5]
Effects in Breastfed Infants
Irritability and fretful sleeping occurred in a 3-day-old breastfed infant on days of maternal aminophylline intake of 200 mg every 6 hours. These effects ceased with discontinuation and recurred on rechallenge over the next 9 months. These effects were probably caused by theophylline in breastmilk. Another five infants reported in this paper showed no adverse reactions after maternal theophylline ingestion.[4] Accumulation of theophylline in infant serum appears most likely in neonates and premature infants because they eliminate theophylline slowly.[3,7]
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Alternate Drugs to Consider
References
- 1.
- National Heart, Lung, and Blood, Institute, National Asthma, Education, and, Prevention, Program, Asthma, and, Pregnancy, Working, Group. NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment-2004 update. J Allergy Clin Immunol 2005;115:34-46. [PubMed: 15637545]
- 2.
- Middleton PG, Gade EJ, Aguilera C, et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J 2020;55:1901208. [PubMed: 31699837]
- 3.
- Stec GP, Greenberger P, Ruo TI, et al. Kinetics of theophylline transfer to breast milk. Clin Pharmacol Ther 1980;28:404-8. [PubMed: 7408400]
- 4.
- Yurchak AM, Jusko WJ. Theophylline secretion into breast milk. Pediatrics 1976;57:518-20. [PubMed: 1264548]
- 5.
- Abduljalil K, Gardner I, Jamei M. Application of a physiologically based pharmacokinetic approach to predict theophylline pharmacokinetics using virtual non-pregnant, pregnant, fetal, breast-feeding, and neonatal populations. Front Pediatr 2022;10:840710. [PMC free article: PMC9150776] [PubMed: 35652056]
- 6.
- Gardner MJ, Schatz M, Cousins L, et al. Longitudinal effects of pregnancy on the pharmacokinetics of theophylline. Eur J Clin Pharmacol 1987;32:289-95. [PubMed: 3595701]
- 7.
- Reinhardt D, Richter O, Brandenburg G. Pharmacokinetics of drugs from the breast-feeding mother passing into the body of the infant, using theophylline as an example. Monatsschr Kinderheilkd 1983;131:66-70. [PubMed: 6843559]
Substance Identification
Substance Name
Theophylline
CAS Registry Number
58-55-9
Drug Class
Breast Feeding
Lactation
Milk, Human
Anti-Asthmatic Agents
Bronchodilator Agents
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- NTP Toxicology and Carcinogenesis Studies of Theophylline (CAS No. 58-55-9) in F344/N Rats and B6C3F1 Mice (Feed and Gavage Studies).[Natl Toxicol Program Tech Rep ...]NTP Toxicology and Carcinogenesis Studies of Theophylline (CAS No. 58-55-9) in F344/N Rats and B6C3F1 Mice (Feed and Gavage Studies).National Toxicology Program. Natl Toxicol Program Tech Rep Ser. 1998 Aug; 473:1-326.
- Review Aminophylline.[Drugs and Lactation Database (...]Review Aminophylline.. Drugs and Lactation Database (LactMed®). 2006
- Bioavailability of four slow-release theophylline formulations in the beagle dog.[J Vet Pharmacol Ther. 1986]Bioavailability of four slow-release theophylline formulations in the beagle dog.Koritz GD, McKiernan BC, Neff-Davis CA, Munsiff IJ. J Vet Pharmacol Ther. 1986 Sep; 9(3):293-302.
- Review Update on the pharmacodynamics and pharmacokinetics of theophylline.[Chest. 1985]Review Update on the pharmacodynamics and pharmacokinetics of theophylline.Hendeles L, Massanari M, Weinberger M. Chest. 1985 Aug; 88(2 Suppl):103S-111S.
- The rise and fall of serum theophylline concentration: a comparison of sustained-release formulations in volunteers with rapid theophylline clearance.[Br J Clin Pharmacol. 1985]The rise and fall of serum theophylline concentration: a comparison of sustained-release formulations in volunteers with rapid theophylline clearance.Mucklow JC, Kuhn S. Br J Clin Pharmacol. 1985 Dec; 20(6):589-96.
- Theophylline - Drugs and Lactation Database (LactMed®)Theophylline - Drugs and Lactation Database (LactMed®)
- eggc.vipupT (0)BioProject
- -eggc.vipnsT (0)BioProject
- -2 eggc.vipExL (21598)BioProject
- eggc.vipwIX (0)BioProject
Your browsing activity is empty.
Activity recording is turned off.
See more...
