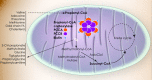
Clinical Description
Propionic acidemia presents with a wide spectrum of symptoms and age of onset. The onset of symptoms in PA varies depending on several factors including residual enzymatic activity, intake of propiogenic precursors, and the occurrence of catabolic stressors. See Table 3a (pdf) and Table 3b (pdf) for a summary of major clinical findings in propionic acidemia (PA) and the reported frequency of symptoms.
Perinatal course. Reported maternal prenatal course, gestational age, and birth length, weight, and head circumference are similar to what is reported for unaffected infants [Kölker et al 2015a]. Increased frequency of miscarriages of affected fetuses is possible [Ottolenghi et al 2010].
Neonatal onset PA. A typical presentation of PA in the neonatal period is characterized by a healthy newborn with poor feeding and decreased arousal in the first few days of life, followed by progressive encephalopathy of unexplained origin. Without prompt diagnosis and management, neonates can develop progressive encephalopathy manifesting as lethargy, seizures, or coma that can result in death (see Table 2). Most individuals eventually diagnosed with PA become symptomatic in the first weeks of life, with 50%-60% exhibiting clinical signs at the time of the newborn screen report [Surtees et al 1992, Dionisi-Vici et al 2006, Grünert et al 2012].
Table 2.
Features of Neonatal-Onset Propionic Acidemia
View in own window
| Clinical Features | Laboratory Findings |
|---|
|
|
|
See Table 3a (pdf) for a summary of the prevalence of major clinical findings during metabolic crisis in propionic acidemia.
Following initial clinical and biochemical stabilization, individuals with neonatal-onset PA may develop a range of symptoms affecting different organ systems. See following and Table 3b (pdf).
Late-onset PA. Residual activity of propionyl-CoA carboxylase may delay the onset of symptoms beyond the neonatal period.
Individuals with late-onset PA may remain asymptomatic and suffer a metabolic crisis under catabolic stress (e.g., illness, surgery, fasting) or experience a more insidious onset with the development of multiorgan complications as summarized in Table 4. See also Table 3b.
Table 4.
Features of Late-Onset Propionic Acidemia
View in own window
| Clinical Features | Laboratory Findings |
|---|
Encephalopathy, coma, &/or seizures precipitated by catabolic stressors (e.g., intercurrent illness, surgery) Vomiting, protein intolerance, failure to thrive, hypotonia, developmental regression, mvmt disorders Isolated cardiomyopathy 1
| ± metabolic acidosis or hyperammonemia ↑ 3-OH propionic acid & methylcitric acid Hyperglycinemia MRI abnormalities incl basal ganglia lesions 2
|
Metabolic decompensations. Children with PA can develop episodic metabolic decompensations, especially in the first years of life. Acidosis, hyperammonemia, pancreatitis, metabolic stroke, cardiomyopathy, bone marrow suppression, seizures, and encephalopathy can accompany acutely deranged metabolism. These episodes can be life-threatening and are often precipitated by illnesses, infections, surgery, or any stress augmenting catabolism. Infectious complications (e.g., sepsis or bacterial meningitis) often accompany metabolic crises and are the major contributors to mortality [Rousson & Guibaud 1984, North et al 1995]. The long-term cognitive outcome of individuals with PA is negatively correlated to the number of metabolic decompensations [Grünert et al 2012].
Growth. Linear growth delay and deceleration of the head circumference may become evident with age and can be seen in both earlier- and late-onset groups [Kölker et al 2015b]. Failure to thrive may be exacerbated by malnutrition secondary to feeding difficulties, recurrent emesis, excessive protein restriction and potentially iatrogenic amino acid imbalances [Manoli et al 2016].
Neurologic manifestations include developmental delay, developmental regression, intellectual disability, seizures, hypotonia, spasticity, and movement disorders [Grünert et al 2012, Pena & Burton 2012, Nizon et al 2013]. Developmental delays and neurologic dysfunction can be seen even in individuals without documented episodes of hyperammonemia or ketoacidosis [North et al 1995, Nyhan et al 1999, Schreiber et al 2012]. The prevalence of intellectual disability can vary between approximately 35% and 76% depending on the reported cohort [de Baulny et al 2005, Dionisi-Vici et al 2006, Touati et al 2006, Grünert et al 2012, Pena & Burton 2012].
Seizures were reported in 13%-53% and EEG abnormalities in 40%-63% of Individuals with PA. Reported forms of seizures included infantile spasms, tonic-clonic, tonic, myoclonic, atonic, absence, and focal [
Haberlandt et al 2009,
Schreiber et al 2012,
Karimzadeh et al 2014,
Kölker et al 2015b]. Seizures were one of the presenting features of the initial metabolic episode in 12%-26% of cases [
Grünert et al 2012,
Kölker et al 2015a].
Psychiatric manifestations. The prevalence of other comorbidities such as attention deficit disorder, autism spectrum disorder, anxiety, and acute psychosis is incompletely characterized [
de Baulny et al 2005,
Pena & Burton 2012,
Nizon et al 2013,
Vernon et al 2014]. Acute psychosis can be a presenting feature of PA in older individuals, especially in those not evaluated by newborn screen, thus warranting a high index of suspicion for this uncommon cause of psychosis in the general population [
Shuaib et al 2012,
Nizon et al 2013,
Dejean de la Bâtie et al 2014].
Brain MRI findings include delayed myelination, white matter changes, basal ganglia abnormalities, cerebellar hemorrhage, and cerebral atrophy [
Schreiber et al 2012]. Clinically unstable individuals appear to be at higher risk of developing brain abnormalities. In a study of 17 PA individuals with clinical seizures, all had abnormal MRI findings and a history of more than ten metabolic decompensations [
Haberlandt et al 2009]. Magnetic resonance spectroscopy (MRS) can reveal decreased myoinositol, N-acetylaspartate and elevated Glx (glutamine, glutamate, and gamma-aminobutyric acid) peaks in basal ganglia [
Bergman et al 1996].
Cardiomyopathy has been recognized as a common complication of PA. Both dilated and hypertrophic cardiomyopathy have been reported [Romano et al 2010]. In the period between 2000 and 2015, its reported prevalence varied between 7% and 24% in various PA cohorts [Dionisi-Vici et al 2006, Romano et al 2010, Grünert et al 2012].
Early clinical manifestations of cardiomyopathy include tachypnea, hepatomegaly, hypotension, tachycardia, or bradycardia.
The age of PA diagnosis, frequency of metabolic decompensation, and residual enzymatic activity do not correlate with presence/absence of cardiomyopathy in individuals with PA [
Romano et al 2010].
Rarely, cardiomyopathy can occur as an apparently
isolated clinical phenomenon in previously healthy individuals without documented episodes of metabolic decompensation or neurocognitive deficits [
Lee et al 2009,
Laemmle et al 2014].
Cardiac rhythm abnormalities. A prolonged QT interval is often detected in individuals with PA [Kölker et al 2015b]. This can be associated with syncope, arrhythmia, and cardiac arrest [Baumgartner et al 2007, Jameson & Walter 2008, Pena & Burton 2012].
Gastrointestinal manifestations
Poor feeding and lack of appetite are common, affecting up to 76% of affected individuals [
Touati et al 2006].
Emesis and diarrhea are commonly reported in individuals with PA, becoming a recurrent problem in approximately 6% [
Kölker et al 2015b].
Liver issues include hepatomegaly, hypoalbuminemia, and abnormal liver function tests (ALT, AST, GGT, INR, and bilirubin) [
Karimzadeh et al 2014,
Kölker et al 2015b]. The etiology of hepatic dysfunction has not been determined with certainty but may include the inherent metabolic derangement as well as cardiac dysfunction in individuals with cardiomyopathy.
Renal abnormalities have been infrequently documented and are likely underreported. Examples have included impaired renal function [Lehnert et al 1994], chronic renal insufficiency leading to renal transplant at age 42 years [Lam et al 2011], and progressive kidney disease in the third decade of life [Vernon et al 2014].
Hematologic abnormalities. Although anemia, leukopenia, and thrombocytopenia are common, pancytopenia is seen less frequently, in 6%-15% of individuals [Grünert et al 2012, Pena & Burton 2012, Karimzadeh et al 2014, Kölker et al 2015b]. Myelodysplastic changes in the bone marrow are uncommon [Stork et al 1986, Sipahi et al 2004].
Immune system. Early retrospective data suggested high frequency of recurrent infections seen in 60%-80% of affected individuals [Lehnert et al 1994, Al Essa et al 1998]. Factors predisposing to infectious complications were likely diverse and included bone marrow suppression, immune dysfunction instigated by propionic acid metabolites, indwelling catheters (e.g., central lines), frequent hospitalizations, and potential nutritional deficiencies caused by dietary modification. Although staphylococcal scalded skin syndrome and Candida skin infections were reported in the earlier literature [Lehnert et al 1994, Al Essa et al 1998], more recent natural history studies suggest that such complications are uncommon [Baumgartner et al 2014, Kölker et al 2015b].
Hypogammaglobulinemia, B-cell lymphopenia, decreased CD4 and CD8 counts, and abnormal CD4/CD8 ratio have been described [Müller et al 1980, Griffin et al 1996, Al Essa et al 1998, Pena & Burton 2012]. Hypogammaglobulinemia, reported in as many as 15% of affected individuals, has required treatment with immunoglobulin in some cases [Müller et al 1980, Raby et al 1994, Pena & Burton 2012].
Ophthalmologic
manifestations. Eye findings include dyschromatopsia, optic atrophy, scotomas, abnormal electroretinogram, visual evoked potentials, and optical coherent tomography. In addition, optic tract and cortical abnormalities have been occasionally noted [Noval et al 2013, Arias et al 2014].
Optic neuropathy occurs in 11%-25% [Pena & Burton 2012, Martinez Alvarez et al 2016]. The onset of optic neuropathy can be acute or insidious; further deterioration can occur during metabolic decompensations triggered by infections or surgery [Noval et al 2013, Martinez Alvarez et al 2016]. The mean age of diagnosis is approximately 13 years (range 2-24 years) [Arias et al 2014, Martinez Alvarez et al 2016].
Hearing loss. Sensorineural hearing loss was reported in 1% and 13% in two large cohorts of individuals with PA [Grünert et al 2012, Kölker et al 2015b].
Musculoskeletal system. Severe osteopenia and osteoporosis have been described in adults with PA [Grünert et al 2012].
Dermatologic manifestations resembling acrodermatitis enteropathica are frequently associated with deficiency of essential amino acids, particularly isoleucine, which can be inadvertently over-restricted in the diet of persons with PA [Domínguez-Cruz et al 2011].
Other rare complications.
Isolated case reports describe clinical findings that could be causally associated with propionic acidemia, but require further characterization: muscle lipidosis [de Baulny et al 2005]; myopathy [Martinez Alvarez et al 2016]; premature ovarian insufficiency [Lam et al 2011]; oligomenorrhea [Martín-Hernández et al 2009]; hypothyroidism [Vernon et al 2014, Martinez Alvarez et al 2016]; parathyroid hormone resistance resolving after hemodialysis [Griffin et al 1996].
Life span. PA confers a high risk of mortality. Reported mortality rates appear to be on the decline: 41%-90% in the 1980-90s, 17%-72% in 2000s, and 7%-12% in early 2010s [Rousson & Guibaud 1984, Surtees et al 1992, van der Meer et al 1996, Pérez-Cerdá et al 2000, Sass et al 2004, de Baulny et al 2005, Dionisi-Vici et al 2006, Touati et al 2006, Grünert et al 2012]. Observed decline in reported mortality likely reflects the length of follow up, introduction of newborn screening, expansion of the PA phenotype, proactive medical management, and elective liver transplantation.
Long-term adult outcomes. Systematic studies of adult individuals with PA are lacking. Early reports suggest that adults with PA can experience significant osteopenia, osteoporosis, renal failure, and premature ovarian failure [Martín-Hernández et al 2009, Lam et al 2011, Vernon et al 2014].
Atypical presentation. Apparently asymptomatic individuals with PA (9%-17% in different cohorts) represent a heterogeneous group consisting of otherwise healthy infants identified after newborn screening, sibs ascertained through evaluation prompted by diagnosis of the proband, and individuals with a diagnosis of PA with incomplete clinical characterization [Grünert et al 2012, Kölker et al 2015a].