NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Forum on Microbial Threats. The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Opportunities for Integrated Intervention Strategies. Washington (DC): National Academies Press (US); 2011.

The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Opportunities for Integrated Intervention Strategies.
Show detailsAbstract
The centenary in 2009 of the discovery of Trypanosoma cruzi and Chagas disease by Brazilian physician Carlos Chagas provided opportunities to revisit the history of this infection and initiated new calls for greater progress in combating this infection and the devastating disease that it causes. Unfortunately, the news on Chagas disease has not been good, as more than 100 years of research and clinical studies have failed to yield consistent and reliable diagnostics, dependable treatment therapies free of side effects, and even a hint of a useful vaccine to prevent infection. Consequently, the number of people infected remains largely a guess and most of these people go untreated due to, among other things, uncertainties about the relative benefits of treatment. Additionally, vector control—long the mainstay of Chagas disease prevention efforts—suffers from underfunding and decentralization of facilities and manpower at the same time as reports surface of insecticide resistance in multiple regions. Undoubtedly, Chagas disease continues to earn the label of “the most neglected of the neglected diseases” (Anonymous, 2006)—understudied, often ignored by funders, and misunderstood by policy makers. Nevertheless, a reexamination of some of the historic dogmas in the field and consideration of new insights and opportunities suggest some bright spots and hope for progress in the future. This review attempts to critically address some of the beliefs that have dominated the Chagas field—and in some cases frustrated progress—in the past 101 years and to highlight some of the encouraging prospects for the near future.
General Background
Trypanosoma cruzi is a hemoflagelate parasite of wildlife, domestic animals, and humans and is the etiological agent of Chagas disease, a chronic affliction that often results in debilitating heart or gut disease. T. cruzi is found throughout much of rural as well as periurban and urban (Ramsey et al., 2005) areas of Latin America as well as in the southern United States. Transmission of T. cruzi is mainly via the contaminated feces left by blood-feeding triatomine insects that inhabit poor-quality housing in Latin America (Cohen and Gurtler, 2001). Infection can also occur congenitally, through blood transfusion or organ transplantation, and through the ingestion of contaminated food or liquids (Benchimol Barbosa, 2006). An oral/mucosal route of infection in humans and other animals is probably more common than is generally appreciated. It is also likely in most cases that infection is initiated with a relatively small number (fewer than hundreds) of parasites, which appear to spread rapidly throughout the host. There is no evidence for the restriction of parasites at the initial infection site or for a significant impact of the infection route on the overall course and outcome of infection.
In mammals, T. cruzi cycles between trypomastigotes that circulate in the blood and amastigotes, a replicative form that resides in the cytoplasm of infected host cells. Amastigotes complete eight or nine rounds of division over a four-to five-day period, eventually emerging from the compromised host cell as trypomastigotes that can reinfect other cells or be acquired by insect vectors during the course of their feeding. In the gut of the triatomine, these ingested trypomastigotes develop into rapidly dividing epimastigotes that move along the gut over several weeks. Upon reaching the hindgut, the parasites differentiate into metacyclic trypomastigotes, a stage similar to the blood-form trypomastigotes and capable of initiating infection in mammals.
Estimates of the impact of T. cruzi infection in the Americas range widely and are undependable because of the absence of routine surveillance and reporting and the relatively poor sensitivity of screening tests. It is likely that between 10 million and 20 million people in Central and South America are infected, making T. cruzi infection the highest-impact infectious disease in this region with yearly losses of more than 50,000 lives and 0.586 million disability-adjusted life-years (Mathers et al., 2006). Bolivia is the most highly affected, with a countrywide infection rate of about 6 percent and reaching 40 percent is some settings (Hidron et al., 2010). At the other end of the spectrum, the United States is estimated to have ~300,000 T. cruzi–infected individuals; some of these autochronous cases are attributed to the presence of the complete transmission cycle (e.g., vectors, parasites, and infected mammals) in most of the southern United States (Beard et al., 2003; Bern and Montgomery, 2009; Dorn et al., 2007; Kjos et al., 2008). The potential for congenital, transfusional, and transplantational mechanisms of transmission have also made T. cruzi infection a significant risk globally, despite the absence of vector transmission outside the Americas (Gascon et al., 2010; Schmunis, 2007).
Because T. cruzi is zoonotic and naturally circulates in more than 100 mammalian species, it will never be eradicated. But its impact on humans can be managed and minimized. The upside of the ability of T. cruzi to infect many different host species is that there are excellent model systems for investigating the complexities of the infection and for testing control and treatment options.
Immunity and Disease
The vast majority of individuals infected with T. cruzi appear to control but not completely eliminate the infection. Severe acute infections may occur in those receiving a high infective dose (apparently the case in some oral infection outbreaks) or in the immunosuppressed. In these cases myocarditis and/or meningoencephalitis are common and may be lethal. Otherwise, acute-stage symptoms are generally rare or benign (e.g., fever, swollen lymph glands, and, occasionally, an inflammatory reaction at the bite site). The transition to a relatively asymptomatic chronic infection (also referred to as the “indeterminate phase”) is marked by the generation of potent immune control of the infection and a consequent decrease in parasite levels. Parasites not only become less abundant in the face of the developing immune responses but also become confined to only certain host tissues (muscle, fat, and nervous system)—not inconsequentially, also the sites of eventual disease. There have been anecdotal reports of spontaneous cure of infection (e.g., a positive serologic response becoming negative over time), but there has not been a systematic study of this phenomenon.
The clinically quiet phase of the chronic infection ends when the cardiac or gastrointestinal complications of chronic Chagas disease start to manifest. The severity of clinical disease is highly variable; in the case of cardiac involvement, chronic T. cruzi infection may result in arrhythmias, apical aneurysm, congestive heart failure, thromboembolism, and sudden death. Chagas cardiomyopathy is the most common cause of cardiomyopathy in South and Central America and the leading cause of cardiovascular death in disease-endemic regions. The fraction of infected individuals who develop clinical symptoms as a result of chronic T. cruzi infection has been estimated to be 30 to 40 percent, although these figures are not well documented and may vary between regions because of the genetic backgrounds of the humans and parasite populations as well as other factors (population age, nutritional status, socioeconomic conditions, exposure to superinfection or co-infections, etc.).
Immune control of T. cruzi infection in most hosts is multidimensional and highly potent (Tarleton, 2007). The numbers of intracellular amastigotes and extracellular trypomastigotes in mammals are tightly controlled by abundant cytolytic CD8+ T cells and ample antibody responses, respectively, both assisted by CD4+ T helper cells that also enhance macrophage-mediated parasite-killing mechanisms. Studies in various immunodeficient mouse strains suggest that the absence of any one of these immune mechanisms results in an uncontrolled, high parasitemic and eventually lethal infection (Tarleton, 2007). One of the persistent dogmas of T. cruzi infection is that it is “immunosuppressive” and fails to generate protective immune responses. However, multiple lines of data do not support this assumption, including (1) the measurement of exceptionally robust antibody and T cell responses in infected hosts, (2) the maintenance of frm control of parasite levels, over a period of decades in humans, and the loss of that control upon chemical or biological immunosuppression, and (3) the ability to transfer relative protection to naïve hosts via immune antibodies and T cells. Despite this strong and effective immune response and its ability to limit the parasite load to nearly undetectable levels, the infection persists in most cases. Like other persistent pathogens, T. cruzi may utilize a number of immune escape mechanisms that make long-term persistence possible. Additionally, long-term persistence itself appears to result in a gradual dwindling of immune function over time because of immune exhaustion (Albareda et al., 2006, 2009).
The origins of clinical disease in human T. cruzi infection are still debated, but it is clear that the decades-long persistent parasitization of the affected muscle tissue is the key element determining disease severity (Tarleton, 2003). Tissue damage probably accumulates slowly over the course of infection, as parasites and the immune responses to them create focal lesions that eventually compromise muscle integrity and function. Qualitative and quantitative aspects of the host immune response could also play an important role in the disease process (i.e., the more efficient the immune response is in controlling parasite replication, the lower the rate of tissue damage and the slower the development of clinical disease). Although highly efficient control of T. cruzi infection may limit disease severity, the associated absence of parasitologic cure raises a number of problems and questions:
- Detection of parasites—the direct evidence of infection—is often very difficult in the chronic phase of infection. This is a key challenge for diagnosis of the infection and an even greater problem for determining the benefits of treatment.
- Disease occurs despite maintenance of very low parasite numbers. An effective treatment or preventative is going to have to do better than the normal immune response (e.g., clear 100 percent of parasites).
- If cure is not possible despite highly effective immune responses, and if superinfection or re-infection following cure is common, what does this tell us about the chances for development of a vaccine that will prevent infection in humans?
The priority issues for Chagas disease include the following:
- Development of better diagnostics;
- Discovery, development, and testing of better treatment regimens, including new drugs;
- Development of better methods for assessing treatment efficacy; and
- Development of integrated, sustainable vector control protocols.
The problems and the needs for each of these issues are discussed in detail below.
Diagnosis
Diagnosis of T. cruzi infection is challenging for a number of reasons. The initial infection is often not detected except in the rare cases of high infective doses and severe acute symptoms (Aguilar et al., 2007; Benchimol Barbosa, 2006; Shikanai-Yasuda et al., 1991), or when inflammation occurs at the site of parasite entry (Nicholls et al., 2007). Although parasites may be visible in the blood during the one- to two-month acute phase, they are difficult to detect thereafter. Amplification techniques (e.g., hemoculture, xenodiagnoses, and polymerase chain reaction [PCR]) have been extensively evaluated as diagnostic tools with highly variable results (reviewed in Cooley et al., 2008). Most studies suggest that fewer than 50 percent of seropositive individuals have detectable parasites or parasite DNA, although numbers at both extremes of this average have been reported. In one of the more herculean attempts to assess the dependability of parasite detection in the chronic phase of T. cruzi infection, Cerisola et al. (1974) used xenodiagnosis (i.e., insect vectors as detectors of T. cruzi) to periodically sample 30 seropositive individuals as many as 21 times over several years, using 80 bugs per time point per subject. Only six subjects consistently had at least one infected bug at each sampling point but the remainder had one or more time points at which none of the 80 insects were positive. In the most extreme case, parasites were detected in only 2 of 18 sampling points (i.e., only 2 of 1,440 bugs fed on this individual were positive). This study firmly documents not only the low parasite levels in chronically infected subjects but also the between- and within-subjects variability in detecting those parasites. This type of sampling error carries over to other amplification methods as well—including PCR—making these techniques instructive when positive but uninformative when negative.
Conclusive diagnosis of T. cruzi infection usually requires positive results on at least two out of three serological tests of different formats (e.g., ELISA, indirect immunofluorescence, hemagglutination, complement fixation, etc.); sera that are positive on only one of three tests are termed “discordant” and these donors are rarely evaluated further or treated. Studies from different geographic regions have documented the undependability of current diagnostics (Avila et al., 1993; Caballero et al., 2007; Castro et al., 2002; Gutierrez et al., 2004; Marcon et al., 2002; Picka et al., 2007; Pirard et al., 2005; Salomone et al., 2003; Silveira-Lacerda et al., 2004; Wincker et al., 1994; Zarate-Blades et al., 2007). For example, Pirard et al. (2005) screened nearly 400 randomly selected blood samples from a Bolivian blood bank and found that 33 percent were positive by all seven of the serological tests employed. However, nearly 20 percent of samples were positive on one or more, but not all seven, tests. Thus, depending on the tests used in a standard “best two-out-of-three” approach, the same individual could be judged to be infected, not infected, or unknown.
This poor state of affairs with respect to diagnosis is in many ways not surprising, given the approaches used in the production of these tests. Many of these serological tests, including one approved for blood screening in the United States (Tobler et al., 2007), use crude or semipurified parasite preparations, often derived from epimastigotes, a stage of T. cruzi present in the invertebrate vector but not in vertebrate hosts. Other tests have incorporated more defined parasite components, including multiple fusion proteins containing epitopes from various parasite proteins, which individually have shown some promise as diagnostics (Caballero et al., 2007; Chang et al., 2006; da Silveira et al., 2001). In the absence of a true gold standard for determining infection status, new tests are usually evaluated using only sera that are positive on multiple other serologic tests, and not sera that are borderline, equivocal, or “discordant.” This approach virtually ensures that the test being evaluated is no worse, but not necessarily any better than, other tests with known limitations.
The acute need in diagnostics is for a rigorously validated test that can conclude positive or negative serological status on the basis of a single assessment and thus without “discordant” results. The format of the test is not crucial; it need not be a rapid or point-of-care test because initiation of treatment following a positive diagnosis is very rarely time-sensitive. The bulk of current screening is done in reference laboratories where speed and simplicity are not the most crucial parameters. It is much more important that the test be accurate, dependable, and preferably quantitative, so that changes in serologic status can be monitored over time, particularly posttreatment. The author's lab has put forward one candidate for an improved diagnostic test for T. cruzi that measures antibodies to a panel of more than a dozen recombinant T. cruzi proteins (Cooley et al., 2008). The proteins included in this multiplex formatted test were selected from nearly 400 candidates using a broad panel of patient sera and includes proteins that are unique to T. cruzi (i.e., not present in other kinetoplastids) as well as proteins that are likely to be highly conserved among parasite isolates from different geographic regions and of different genetic types.
Drug Discovery, Testing, and Use
Two nitroaromatic heterocycle compounds are currently available for treatment of T. cruzi infection: benznidazole (BZ, Rochagan®, Roche Pharmaceuticals) and nifurtimox (NX, Lampit®, Bayer Healthcare). NX acts via the reduction of the nitro group to a nitroanion radical that reacts with oxygen to produce toxic superoxide anions (Docampo and Stoppani, 1979; Docampo et al., 1981). The mechanism of action of BZ also appears to involve nitro reduction, but the reduced intermediates are thought to act by covalently modifying various biomacromolecules (Docampo, 1990; Moreno et al., 1982). Both drugs have significant side effects that limit their use and effectiveness.
Another unfortunate but widely accepted dogma of Chagas disease is that these drugs “cure” most acute infections but are ineffective in treating the chronic phase infection. This conclusion is based upon evidence of posttreatment conversion to negative serology (and in some cases, negative parasitemia) in acute and shorter-length infections and the relative lack of such evidence in chronically infected adults. Additionally, the fact that some of these results come from randomized, placebo-controlled trials in the case of children and adolescents (Andrade, 1996; Sosa Estani, 1998), but that such trials have not been conducted in adults (although several such studies are nearing completion; Marin-Neto, 2008) is often cited as evidence of the ineffectiveness of these drugs in longer-term infections. The latter is clearly an instance of “absence of proof” not being “proof of absence.” In reality there is little direct proof of “cure” resulting from treatment in either children or adults. As already noted, a reduction in the detection of parasites or parasite products in the circulation is not proof of cure. Conversion to negative serology is a reasonable and accepted, though unproven, surrogate for cure. That conversion to a negative serology is more common following treatment of acute or shorter-term infections is not surprising because the B cell memory and antibody levels achieved during a chronic infection would be expected to be slower to decline as compared to that of an acute or shorter-term infection in younger subjects. Furthermore, the multiplex serological test mentioned above detects declines in antibody levels in BZ-treated adults that are rarely evident using conventional serology, suggesting that better tools may provide better data on the outcome of treatment (Laucella et al., 2009). In short, clear and convincing evidence for treatment effectiveness is hardly any worse in adults than in children (or in chronic than in acute infections, as this is often interpreted), making the bias against treating those with chronic infection unfounded, at least based upon this body of evidence.
The biology of T. cruzi and the features of the infection also provide no support for a differential susceptibility to treatment during the acute and chronic phases; parasites are not quiescent in the chronic phase but rather continue to cycle in and out of host cells, to replicate and metabolize, and thus presumably are equally susceptible to antimicrobials irrespective of the length of the infection. Most important, the substantial evidence from observational studies of long-term posttreatment follow-up of chronic-stage treatment provides unequivocal verification of the ability of treatment to substantially impact disease progression (reviewed in Tarleton et al., 2007). Although these latter studies may be criticized for their nonrandomized design, their conclusions should not simply be discarded on this basis.
Despite the evidence for the efficacy of BZ and NX, these are far from ideal drugs. Both have substantial, although often manageable (Viotti et al., 2009), side effects, and treatment failure occurs in a variable number of cases. The biochemical basis of treatment failure is not fully understood; there are isolates of T. cruzi that are naturally more resistant to these compounds (both in vitro and in vivo) and in some cases this increased resistance is associated with decreased nitroreductase activity in these isolates (Wilkinson et al., 2008). The combination of misinformation about the efficacy of treatment in the chronic phase, the possibility of adverse effects from treatment, the lack of reliable methods to assess treatment efficacy, and the known variability in efficacy and the undependable supply of compounds virtually ensure that these drugs will continue to be profoundly underutilized, despite their effectiveness. Because drug treatment is the only effective means of preventing the development of clinical disease, it should be used in all acute and chronic infection cases where side effects or other aspects of the treatment do not put the patients at a greater health risk. It is unethical not to make better use of the tools that we already have in hand, and access to treatment, not only in Latin America but throughout the world, must be improved (Gascon et al., 2010).
There have been no new drugs developed for the treatment of T. cruzi infection in decades and the investigations of compounds as potential treatments nearly always stop well short of demonstration of parasitological cure in animals models. Fortunately, this is one of the areas that show real promise for rapid progress in the coming years. With respect to compound discovery, excellent systems are now available for high-capacity in vitro screening of compound libraries, rapid in vivo compound testing, and rigorous analysis of cure in both acute and chronic infections in experimental hosts (Figure A21-1) (Canavaci, 2010). Highthroughput screens of large compound libraries (containing from 300,000 to more than 1,000,000 compounds) have been completed or are in progress. And plans have been announced to conduct two clinical trials of new compounds, the already licensed antifungal posaconazole by Merck and the ergosterol biosynthesis inhibitor ravuconazole pro-drug E1224 by the Drugs for Neglected Diseases Initiative (DNDi) and Eisai. This progress is being made possible by a combination of the persistence of individual investigators who have developed the testing protocols and provided the initial discovery data for the compounds going to clinical trials, large pharmaceutical companies who have the compound libraries, testing capacity, and chemical expertise, and public–private partnerships like DNDi that have helped coordinate some of these efforts.
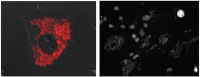
FIGURE A21-1
Amastigotes of T. cruzi within host cells. (left) CL strain parasites expressing the tandom Tomato red protein and useful for both in vitro and in vivo screening assays (Canavaci et al., 2010). (right) Example of high-content microscopic screening using (more...)
The interest of the pharmaceutical industry in Chagas disease is exciting, but this interest could wane as quickly as it has come, especially if the initial clinical trials are not promising; there may be limited tolerance for failure. Funding for these efforts is also still tenuous; industry is donating resources but will likely need partners and other funders to get new compounds approved and to the clinic. Preclinical studies must rigorously evaluate efficacy using the best available model systems that also allow for definitive conclusions and comparative data between different candidate drugs. With these data in hand, the various entities can make coordinated and informed decisions, conserving resources by ensuring that only the best compounds go forward in development.
Assessing Treatment Efficacy
A principal consequence of the highly effective immune control of parasite load in T. cruzi infection is that detection of parasites or parasite products is challenging in the absence of treatment and totally unreliable as a measure of effectiveness following treatment; a positive parasitemia or PCR-indicated treatment failure but a negative test does not indicate successful cure. Given this fact, the development of a test that absolutely certifies parasitological cure following patient treatment is going to be difficult, if not impossible. However, this is not a situation that is unique to Chagas disease and is no justification for not treating using the current drugs or for not developing and testing new treatments. Surrogates of cure will likely have to be the principal metric for treatment outcomes. Two measures have been used extensively to assess treatment success: decreases in the titers of anti–T. cruzi antibodies (Andrade, 1996; Sosa Estani et al., 1998) and the prevention of progression of symptomatic disease (Viotti et al., 1998). Both criteria are useful on a population basis to demonstrate the benefit of therapy and, on an individual basis, conversion to negative serology is a convincing indicator of parasitological cure. However, both outcomes take years (or even decades) of observation (e.g., the rate of progression to more severe disease is estimated at ~3 percent of subjects per year; Pinto Dias, 2006). A decade-long follow-up period is not an acceptable endpoint for the testing of new drugs.
Development of antipathogen immune responses is an accepted marker of infection—indeed the basis of diagnosis for many infections—including T. cruzi. A decline in these immune responses can also reflect the clearance of the infection. The maintenance of antipathogen T cell and antibody responses long after infection cure, an important characteristic of an effective immune response, considerably complicates the use of immunological parameters to monitor cure. However, careful examination of the characteristics of these responses during infection and following infection clearance suggests some distinctive aspects that may be useful in assessing cure. For example, by analyzing the antibody responses to multiple, individual T. cruzi proteins (Cooley et al., 2008), posttreatment changes that are not evident from conventional serological tests can be detected within one year after treatment (Laucella et al., 2009). Because these decreased antibody levels are not observed in untreated individuals and occur at a rate that is similar to the rate of cure as assessed by long-term follow-up of progression in clinical disease in drug-treated subjects (Viotti et al., 1994, 2006), this assay appears to be an excellent candidate for further evaluation as an indicator of treatment efficacy.
The attributes of antipathogen T cell responses also follow a predictable pattern after infection cure, with persisting T cells acquiring the phenotype of long-term (central) memory cells and with the loss of effector and shorter-lived effector memory T cells when antigen is no longer in the system (Wherry et al., 2004). A decline in effector T cells specific for T. cruzi has been documented in BZ-treated subjects and strongly correlates with decreasing antibody levels (Laucella et al., 2009). T cell responses are more cumbersome to measure than are serologic responses and are sometimes undetectable in subjects even before treatment (Laucella et al., 2009), making this a less dependable marker for cure. Nevertheless, these studies support further investigations of immunological parameters as possible markers of treatment efficacy in T. cruzi infection. These and other biomarkers of treatment success are likely to become the primary endpoints for clinical trials of new drugs for treating T. cruzi infection. None of these surrogate markers is likely to be directly confirmable as an indicator of cure (i.e., we cannot immunosuppress human subjects posttreatment to confirm cure, as is done in experimental models; Bustamante et al., 2008). Upcoming clinical trials, where controlled follow-up and multiple endpoints and outcomes will be measured, should be used as opportunties to evaluate some possible surrogate markers.
It could be argued that drug treatment in Chagas disease could be efficacious simply by decreasing parasite load and, consequently, the level of inflammation and tissue damage, even if it fails to completely clear T. cruzi infection. Although treatment without cure could be beneficial, most data argue against this possibility. First, disease development is clearly linked to parasite persistence: as long as parasites are present, there is potential for more tissue destruction. Also, subjects with low and even undetectable parasite load still go on to develop clinical disease. Indeed, there is no evidence of an association between disease severity and parasite load (Hidron et al., 2010; Murcia, 2010). Finally, a drop in parasite load brought about by drug treatment would be expected to be only temporary and last only as long as the drug is being given. It is possible that a new, lower set-point of parasite load would be established after treatment, but this does not seem to be consistently the case in either human (Murcia et al., 2010) or experimental infections (Bustamante and Tarleton, unpublished). Also, the changing nutritional, general health, and especially immunological status of infected subjects would be expected to modify the efficiency of infection control overtime. In short, lowering parasite levels is not a dependable and acceptable goal for drug treatment in T. cruzi infection; the objective needs to be parasitological cure.
Vector Control
Without question, the biggest success story in the control and prevention of Chagas disease has been vector control. Triatoma infestans is the vector species responsible for the majority of T. cruzi transmission to humans in South America and is found almost exclusively in and around housing, living in the cracks and crevices of adobe, mud, and thatch constructions, and feeding at night on the animal and human inhabitants (Figure A21-2). Widespread, consistent, and highly effective insecticidal spraying campaigns in the 1980s and 1990s, focusing largely on this domiciliary vector species, dramatically reduced incidence of T. cruzi infection in the area known as the Southern Cone of South America. As a result, Brazil, Uruguay, and Chile were declared free of transmission by T. infestans (Moncayo and Silveira, 2009) and the Pan American Health Organization and the World Health Organization set their sights on “elimination” of T. infestans–mediated transmission of T. cruzi by 2010.

FIGURE A21-2
A setting of active transmission in the Gran Chaco region and the pyrethroid-resistant Triatoma infestans collected from the structure.
Such goals perpetuate another myth of T. cruzi infection—that vector transmission can be eliminated by insecticidal spraying alone. There is a long list of reasons that this is highly unlikely if not simply impossible. First, insecticidal spraying is time-consuming, labor-intensive, and expensive; multiperson crews must remove all belongings from structures before spraying the walls and roofs with residual insecticides. And this process has to be repeated every six months, perhaps forever, to eliminate reinfestations. The well-funded national campaigns that made the Southern Cone Initiative successful have now been largely dismantled, and the responsibility of vector control has fallen to underfunded, underequipped, and understaffed local governments (Gurtler et al., 2008). Second, T. infestans is not the only vector for T. cruzi and is not exclusively domiciliary. A dozen or more species of reduviid bugs are likely capable of vectoring T. cruzi infection, and these species each have unique behaviors and distribution patterns and thus distinctive ways of interfacing with humans. Discovery of sylvatic foci of T. infestans indicates that this species will not be eliminated as a transmission threat even if it is removed from all domestic dwellings by insecticide use (Noireau et al., 2005). Additionally, new settings for transmission in and near cities are making it clear that T. cruzi transmission is not restricted solely to rural settings (Bowman et al., 2008). Third, and the least surprising facet, resistance to insecticides is being reported in multiple settings (Figure A21-2) (Picollo, 2005). Whether this resistance is due exclusively to the decades of house spraying or if the agricultural use of insecticides is also contributing is not known. There are alternative insecticides, but these are often more expensive and are too noxious to the inhabitants of houses to be widely accepted.
Thus, although vector control by insecticide use has been an unqualified success, it is not a long-term solution, particularly when applied in isolation from other vector transmission and infection control tools. The unique behavioral characteristics of various vector species and the increasing variety of settings in which transmission is being reported emphasizes that one size does not fit all when it comes to dealing with the vectors of T. cruzi and with transmission control in general. For example, in the Grand Chaco region of Northern Argentina, companion animals, not humans, have been identified as the major infection source of bugs that subsequently transmit the infection to the human residents of the house (Cohen and Gurtler, 2001). Recent outbreaks resulting from apparent oral transmission are often presented as evidence of a “new” route of transmission (Aguilar et al., 2007; Benchimol Barbosa, 2006; Shikanai-Yasuda et al., 1991). More likely, oral transmission is a common, if not the dominant, route of transmission in humans that is just now being more widely recognized. The transmission characteristics in different environments have to be better studied, and integrated plans for each specific local situation must be designed. In this process, there needs to be better use of simple tools where effective (e.g., bug collars on domestic animals and insecticidal screens and nets on houses and beds), along with the evaluation of more innovative approaches, such as the vaccination or intermittent treatment of companion animals to prevent them from being sources of infection. The identification and aggressive treatment of parasitemic humans, especially the minority of “supertransmitters” who are highly infective for insects, is needed (Cerisola et al., 1974). The development of cheap house construction methods and materials that discourage infestations is a clear long-term solution to permanently decrease the opportunities for human infection. Finally, an improved infrastructure for and commitment to supporting vector and transmission control in all countries of the Americas is needed.
Summary: Leadership and Policy Making
Although the problems are many, the outlook for making an impact on Chagas disease is nonetheless bright. This is not a difficult infection to understand; the vectors are large insects that primarily feed within a house and that transmit the infection indirectly and inefficiently via their feces, not their bite. Infection rarely kills acutely, so there is plenty of time to treat the infection and the goal of treatment—to eradicate, not manage the infection—is obvious, even if difficult to certify. Current diagnostics are usable but need improvement, particularly with respect to assessing treatment efficacy. Drugs are available that are effective in many cases. Because these are the only option for the 20 million individuals already infected and for those who will become infected in the future, these should be more widely used despite their potential side effects. The explosion in interest in new drug development in the past few years and announced plans for clinical trials are extremely encouraging that safer drugs are on the horizon. Vector control has already shown its utility; it just needs to be conducted more intelligently and with better integration with other treatment and preventative programs. Not discussed above are the opportunities for a human vaccine for Chagas disease; the jury is still out on this possibility. Given the perceived requirement that elimination and not simply better control of an existing infection is the goal, prophylactic or therapeutic vaccines would have to totally prevent infection or promote complete parasite clearance in those already infected. This is a big task for a vaccine. Until such abilities can be demonstrated in experimental infections, vaccines for T. cruzi will likely remain just a hope.
A particularly vexing problem in Chagas disease is in the areas of leadership and policy. National (including in the United States) and international policies have been ineffective at best, harmful at worst. Perpetuating the myths that chronic Chagas disease cannot be treated with current drugs and that transmission can (or has) been largely eliminated does not accurately reflect the bulk of the data. Policies that rely almost exclusively on insecticidal spraying and speak of eradication/elimination within years when this is impossible, no matter what the time frame, is careless. These practices minimize the severity of the problem, obstruct the use of current drugs and the development of new ones, encourage the elimination or decentralization of vector control programs, and discourage the involvement of large nonprofit funders in new or continuing control and prevention efforts. A policy of “more of the same” will not achieve the progress that is necessary and possible. National and international policy makers have to do a much better job at honestly assessing the problems and the realistic opportunities and coordinating the effort to build upon these. Establishment of research and development priorities based upon rigorous and educated evaluations and developing the funding mechanisms to move beyond planning to implementation is crucial for making real progress. Success will require not only funding but also political will and local buy-in. Scientists must execute the appropriate studies and provide the data and clear interpretations that can guide policy development and implementation. T. cruzi infection and Chagas disease are manageable problems—there is a success story waiting to be written here if the job is done carefully and correctly.
References
- Aguilar HM, Abad-Franch F, Dias JC, Junqueira AC, Coura JR. Chagas disease in the Amazon Region. Memórias do Instituto Oswaldo Cruz. 2007;102(Suppl 1):47–56. [PubMed: 17891274]
- Albareda MC, Laucella SA, Alvarez MG, Armenti AH, Bertochi G, Tarleton RL, Postan M. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas' disease patients. International Immunology. 2006;18(3):465–471. [PubMed: 16431876]
- Albareda MC, Olivera C, Laucella S, Alvarez MG, Fernandez ER, Lococo B, Viotti R, Tarleton R, Postan M. Chronic human infection with T. cruzi drives CD4+ T cells to immune senescence. Journal of Immunology. 2009;183(6):1675–1684. [PMC free article: PMC3074976] [PubMed: 19692645]
- Anonymous. Chagas' disease—an epidemic that can no longer be ignored. Lancet. 2006;368(619) [PubMed: 16920444]
- Avila HA, Pereira JB, Thiemann O, De PE, DeGrave W, Morel CM, Simpson L. Detection of Trypanosoma cruzi in blood specimens of chronic chagasic patients by polymerase chain reaction amplification of kinetoplast minicircle DNA: Comparison with serology and xenodiagnosis. Journal of Clinical Microbiology. 1993;31(9):2421–2426. [PMC free article: PMC265772] [PubMed: 8408566]
- Beard CB, Pye G, Steurer FJ, Rodriguez R, Campman R, Peterson AT, Ramsey J, Wirtz RA, Robinson LE. Chagas disease in a domestic transmission cycle, southern Texas, USA. Emerging Infectious Diseases. 2003;9(1):103–105. [PMC free article: PMC2873735] [PubMed: 12533289]
- Benchimol Barbosa PR. The oral transmission of Chagas' disease: An acute form of infection responsible for regional outbreaks. International Journal of Cardiology. 2006;112(1):132–133. [PubMed: 16600406]
- Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clinical Infectious Diseases. 2009;49(5):e52–e54. [PubMed: 19640226]
- Bowman NM, Kawai V, Levy MZ, Cornejo del Carpio JG, Cabrera L, Delgado F, Malaga F, Cordova Benzaquen E, Pinedo VV, Steurer F, Seitz AE, Gilman RH, Bern C. Chagas disease transmission in periurban communities of Arequipa, Peru. Clinical Infectious Diseases. 2008;46(12):1822–1828. [PubMed: 18462104]
- Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nature Medicine. 2008;14(5):542–550. [PMC free article: PMC3074975] [PubMed: 18425131]
- Caballero ZC, Sousa OE, Marques WP, Saez-Alquezar A, Umezawa ES. Evaluation of serological tests to identify human Trypanosoma cruzi infection and cross-reactivity with Trypanosoma rangeli and Leishmania spp cases. Clinical and Vaccine Immunology. 2007;14(8):1045–1049. [PMC free article: PMC2044488] [PubMed: 17522327]
- Canavaci AM, Bustamante JM, Padilla AM, Perez Brandan CM, Simpson LJ, Xu D, Boehlke CL, Tarleton RL. In vitro and in vivo high-throughput assays for the testing of anti-Trypanosoma cruzi compounds. PLoS Neglected Tropical Diseases. 2010;4(7):e740. [PMC free article: PMC2903469] [PubMed: 20644616]
- Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvao LM. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitology Research. 2002;88(10):894–900. [PubMed: 12209329]
- Cerisola JA, Rohwedder R, Segura EL, Del Prado CE, Alvarez MG, Wynne de Martini GJ. El Xenodiagnostico: Normalizacion, Utilidad. Buenos Aires: 1974.
- Chang CD, Cheng KY, Jiang LX, Salbilla VA, Haller AS, Yem AW, Bryant JD, Kirchhoff LV, Leiby DA, Schochetman G, Shah DO. Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion. 2006;46(10):1737–1744. [PubMed: 17002630]
- Cohen JE, Gurtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001;293(5530):694–698. [PubMed: 11474111]
- Cooley G, Etheridge RD, Boehlke C, Bundy B, Weatherly DB, Minning T, Haney M, Postan M, Laucella S, Tarleton RL. High throughput selection of effective serodiagnostics for Trypanosoma cruzi infection. PLoS Neglected Tropical Diseases. 2008;2(10):e316. [PMC free article: PMC2556098] [PubMed: 18841200]
- da Silveira JF, Umezawa ES, Luquetti AO. Chagas disease: Recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends in Parasitology. 2001;17(6):286–291. [PubMed: 11378036]
- Docampo R. Sensitivity of parasites to free radical damage by antiparasitic drugs. Chemico-Biological Interactions. 1990;73(1):1–27. [PubMed: 2406032]
- Docampo R, Stoppani AO. Generation of superoxide anion and hydrogen peroxide induced by nifurtimox in Trypanosoma cruzi. Archives of Biochemistry and Biophysics. 1979;197(1):317–321. [PubMed: 232403]
- Docampo R, Moreno SN, Stoppani AO, Leon W, Cruz FS, Villalta F, Muniz RF. Mechanism of nifurtimox toxicity in different forms of Trypanosoma cruzi. Biochemical Pharmacology. 1981;30(14):1947–1951. [PubMed: 7023488]
- Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, Diaz J, Wesson D. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerging Infectious Diseases. 2007;13(4):605–607. [PMC free article: PMC2725963] [PubMed: 17553277]
- Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Tropica. 2010;115(1–2):22–27. [PubMed: 19646412]
- Gurtler RE, Diotaiuti L, Kitron U. Commentary: Chagas disease: 100 years since discovery and lessons for the future. International Journal of Epidemiology. 2008;37(4):698–701. [PMC free article: PMC2507862] [PubMed: 18653505]
- Gutierrez R, Angulo VM, Tarazona Z, Britto C, Fernandes O. Comparison of four serological tests for the diagnosis of Chagas disease in a Colombian endemic area. Parasitology. 2004;129(Pt 4):439–444. [PubMed: 15521632]
- Hidron AI, Gilman RH, Justiniano J, Blackstock AJ, Lafuente C, Selum W, Calderon M, Verastegui M, Ferrufino L, Valencia E, Tornheim JA, O'Neal S, Comer R, Galdos-Cardenas G, Bern C. Chagas cardiomyopathy in the context of the chronic disease transition. PLoS Neglected Tropical Diseases. 2010;4(5):e688. [PMC free article: PMC2872643] [PubMed: 20502520]
- Kjos SA, Snowden KF, Craig TM, Lewis B, Ronald N, Olson JK. Distribution and characterization of canine Chagas disease in Texas. Veterinary Parasitology. 2008;152(3–4):249–256. [PubMed: 18255233]
- Laucella SA, Perez Mazliah D, Bertocchi G, Alvarez MG, Cooley G, Viotti R, Albareda MC, Lococo B, Postan M, Armenti A, Tarleton RL. Changes in Trypanosoma cruzi-specific immune responses following treatment: Surrogate markers of treatment efficacy. Clinical Infectious Diseases. 2009;49(11):1675–1684. [PMC free article: PMC2805187] [PubMed: 19877967]
- Marcon GE, Andrade PD, de Albuquerque DM, Wanderley Jda S, de Almeida EA, Guariento ME, Costa SC. Use of a nested polymerase chain reaction (N-PCR) to detect Trypanosoma cruzi in blood samples from chronic chagasic patients and patients with doubtful serologies. Diagnostic Microbiology and Infectious Disease. 2002;43(1):39–43. [PubMed: 12052627]
- Mathers CD, Lopez A, Murray A. The burden of disease and mortality by condition: Data, methods, and results for the year 2001, Global burden of disease and risk factors. Lopez CMA, Ezzati M, Jamison D, Murray C, editors. New York: Oxford University Press; 2006. [PubMed: 21250373]
- Moncayo A, Silveira AC. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Memórias do Instituto Oswaldo Cruz. 2009;104(Suppl 1):17–30. [PubMed: 19753454]
- Moreno SN, Docampo R, Mason RP, Leon W, Stoppani AO. Different behaviors of benznidazole as free radical generator with mammalian and Trypanosoma cruzi microsomal preparations. Archives of Biochemistry and Biophysics. 1982;218(2):585–591. [PubMed: 6297399]
- Murcia L, Carrilero B, Munoz MJ, Iborra MA, Segovia M. Usefulness of PCR for monitoring benznidazole response in patients with chronic Chagas' disease: A prospective study in a non-disease-endemic country. Journal of Antimicrobial Chemotherapy. 2010;65(8):1759–1764. [PubMed: 20542903]
- Nicholls RS, Cucunuba ZM, Knudson A, Florez AC, Montilla M, Puerta CJ, Pavia PX. [Acute Chagas disease in Colombia: a rarely suspected disease. Report of 10 cases presented during the 2002–2005 period] Biomedica. 2007;27(Suppl 1):8–17. [PubMed: 18154241]
- Noireau F, Cortez MG, Monteiro FA, Jansen AM, Torrico F. Can wild Triatoma infestans foci in Bolivia jeopardize Chagas disease control efforts? Trends in Parasitology. 2005;21(1):7–10. [PubMed: 15639733]
- Picka MC, Meira DA, de Carvalho TB, Peresi E, Marcondes-Machado J. Definition of a diagnostic routine in individuals with inconclusive serology for Chagas disease. Brazil Journal of Infectious Disease. 2007;11(2):226–233. [PubMed: 17625767]
- Pinto Dias JC. The treatment of Chagas disease (South American trypanosomiasis). Annals of Internal Medicine. 2006;144(10):772–774. [PubMed: 16702594]
- Pirard M, Iihoshi N, Boelaert M, Basanta P, Lopez F, Van der Stuyft P. The validity of serologic tests for Trypanosoma cruzi and the effectiveness of transfusional screening strategies in a hyperendemic region. Transfusion. 2005;45(4):554–561. [PubMed: 15819677]
- Ramsey JM, Alvear AL, Ordonez R, Munoz G, Garcia A, Lopez R, Leyva R. Risk factors associated with house infestation by the Chagas disease vector Triatoma pallidipennis in Cuernavaca metropolitan area, Mexico. Medical and Veterinary Entomology. 2005;19(2):219–228. [PubMed: 15958028]
- Salomone OA, Basquiera AL, Sembaj A, Aguerri AM, Reyes ME, Omelianuk M, Fernandez RA, Enders J, Palma A, Barral JM, Madoery RJ. Trypanosoma cruzi in persons without serologic evidence of disease, Argentina. Emerging Infectious Diseases. 2003;9(12):1558–1562. [PMC free article: PMC3034320] [PubMed: 14720396]
- Schmunis GA. Epidemiology of Chagas disease in non-endemic countries: The role of international migration. Memórias do Instituto Oswaldo Cruz. 2007;102(Suppl 1):75–85. [PubMed: 17891282]
- Shikanai-Yasuda MA, Marcondes CB, Guedes LA, Siqueira GS, Barone AA, Dias JC, Amato Neto V, Tolezano JE, Peres BA, Arruda Junior ER, et al. Possible oral transmission of acute Chagas' disease in Brazil. Revista do Instituto de Medicina Tropical de São Paulo. 1991;33(5):351–357. [PubMed: 1844961]
- Silveira-Lacerda EP, Silva AG, Junior SF, Souza MA, Kesper N, Botelho-Filho A, Umezawa ES. Chagas' disease: Application of TESA-blot in inconclusive sera from a Brazilian blood bank. Vox Sanguinis. 2004;87(3):204–207. [PubMed: 15569074]
- Sosa Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. American Journal of Tropical Medicine & Hygiene. 1998;59(4):526–529. [PubMed: 9790423]
- Tarleton RL. Chagas disease: A role for autoimmunity? Trends in Parasitology. 2003;10:447–451. [PubMed: 14519582]
- Tarleton RL. Immune system recognition of Trypanosoma cruzi. Current Opinions in Immunology. 2007;19(4):430–434. [PubMed: 17651955]
- Tarleton RL, Reithinger R, Urbina JA, Kitron U, Gurtler RE. The challenges of Chagas disease—grim outlook or glimmer of hope. PLoS Medicine. 2007;4(12):e332. [PMC free article: PMC2222930] [PubMed: 18162039]
- Tobler LH, Contestable P, Pitina L, Groth H, Shaffer S, Blackburn GR, Warren H, Lee SR, Busch MP. Evaluation of a new enzyme-linked immunosorbent assay for detection of Chagas antibody in US blood donors. Transfusion. 2007;47(1):90–96. [PubMed: 17207235]
- Viotti R, Vigliano C, Armenti H, Segura E. Treatment of chronic Chagas' disease with benznidazole: Clinical and serologic evolution of patients with long-term follow-up. American Heart Journal. 1994;127(1):151–162. [PubMed: 8273735]
- Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: A nonrandomized trial. Annals of Internal Medicine. 2006;144(10):724–734. [PubMed: 16702588]
- Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Review of Anti-Infective Therapy. 2009;7(2):157–163. [PubMed: 19254164]
- Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proceedings of the National Academy of Sciences U S A. 2004;101(45):16004–16009. [PMC free article: PMC524220] [PubMed: 15505208]
- Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proceedings of the National Academy of Sciences U S A. 2008;105(13):5022–5027. [PMC free article: PMC2278226] [PubMed: 18367671]
- Wincker P, Britto C, Pereira JB, Cardoso MA, Oelemann W, Morel CM. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. American Journal of Tropical Medicine and Hygiene. 1994;51(6):771–777. [PubMed: 7810810]
- Zarate-Blades CR, Blades N, Nascimento MS, da Silveira JF, Umezawa ES. Diagnostic performance of tests based on Trypanosoma cruzi excreted-secreted antigens in an endemic area for Chagas' disease in Bolivia. Diagnostic Microbiology and Infectious Disease. 2007;57(2):229–232. [PubMed: 17020793]
- CHAGAS DISEASE IMPACT AND OPPORTUNITIES: BEYOND THE HISTORICAL DOGMA - The Cause...CHAGAS DISEASE IMPACT AND OPPORTUNITIES: BEYOND THE HISTORICAL DOGMA - The Causes and Impacts of Neglected Tropical and Zoonotic Diseases
Your browsing activity is empty.
Activity recording is turned off.
See more...