This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Show detailsContinuing Education Activity
A macular hole is a clinical condition of the central retina, which leads to the gross impairment of central vision. Many ocular conditions may predispose the eye to the development of a macular hole. Evaluation of macular hole includes the diagnosis of macular hole, along with associated pathologies using modern diagnostic tools and prognosticating individual cases. Proper surgical techniques with advanced technologies, intraoperative maneuvers, and proper postoperative rehabilitation give better anatomical and functional outcomes. This activity reviews the evaluation and management of macular hoe and highlights the role of the interprofessional team in evaluating and improving care for patients with this condition.
Objectives:
- Identify the etiology of macular holes.
- Describe the clinical findings associated with macular holes.
- Outline the appropriate treatment plan for macular holes with proper rehabilitation for better visual outcomes.
- Identify the importance of improving care coordination among interprofessional team members to improve outcomes for patients with macular holes.
Introduction
Macular hole (MH) is a vitreoretinal interface disease characterized by a partial or full-thickness neurosensory retinal defect in the center of the macula. Macular hole, per se, has not been studied a lot up to the early nineties. The diagnosis and treatment plan has been dramatically changed in the last two decades. A new classification system based on morphology and vitreoretinal interface pathology has convincingly revealed the pathways related to the formation of a macular hole.[1] The diagnosis and follow-up after treatment, clinically as well as by optical coherence tomography (OCT), has become definitive nowadays.[2] Due to central foveal involvement, metamorphopsia and visual deprivation are the common presenting symptoms that may be reversible after successful anatomical closure following surgery.
Etiology
A macular hole is mostly idiopathic or related to vitreomacular traction syndrome. The macular hole may be associated with various forms of macular pathology including:
- After laser treatment[3]
- Intraocular surgical intervention[4]
- Epiretinal membrane
- Polypoidal choroidal vasculopathy (PCV)[5]
- Hypertensive retinopathy (HTNR)
- Diabetic retinopathy (DR)
- Vitelliform dystrophy[6]
- Ruptured retinal arterial macroaneurysm (RAM)[7]
Other rare associations are
- Central retinal artery occlusion (CRAO)[8]
- Retinitis pigmentosa (RP)[9]
- Alport syndrome[10]
- Valsalva retinopathy[11]
- Stargardt disease[12]
- Syphilis
Apart from this, ocular pathology, which may predispose the eye to develop a macular hole, is myopia with or without posterior staphyloma.[13] Retinal detachment from the macular hole is not very uncommon, and in these cases, different techniques of hole closure are now being tried.[13][14] Trauma is another important cause, specifically in the young age group.[15] A traumatic macular hole may close spontaneously and, if not, may require surgery.[16] If it is not associated with any other predisposing condition, it is called a primary macular hole. Those associated with predisposing conditions designated as secondary macular holes.
Epidemiology
An idiopathic macular hole is usually unilateral to present. Bilateral involvement varies widely from 2% to 28% though no definitive systemic association was reported.[17][18] Females are more commonly involved (F:M=3:1, range 1.2:1 to 7:1) in their sixth or seventh decade of life.[19][20] Though the mean age is above sixty years, a myopic and traumatic macular hole can present at any age. The prevalence of macular holes can vary from 3.3 per 1000 population (Baltimore eye study) to 0.2 per 1000 population in Australia.[20] The incidence of the macular holes was 7.8 persons per 100000 population per year in a study conducted in the U.S.A.[20] In another study, the cumulative incidence of the macular holes was noted to be 41.1 cases per 100000 person-years.[21] Different systemic risk factors were studied regarding association with macular hole, but it was unrewarding.[22]
Pathophysiology
The exact pathophysiology behind the development of idiopathic macular holes is unknown. But recent advances in the section of ocular diagnostics in the form of spectral-domain optical coherence tomography (SD-OCT) have made a big impact. Vitreo-retinal interface abnormality along with its tractional forces play a major role in the development of macular holes. Involutional foveal thinning also plays a role.[23]
This tractional force may be tangential traction by pre-existing epi-retinal membrane or vertical by vitreomacular traction.[24] This abnormal tangential vitreous traction is the product of fluid movement in the vitreous with cellular proliferation suggested by a study conducted by W.R.Green.[25]
The successful anatomical closure of the macular hole after surgical removal of the vitreous indirectly proves its pathologic role. Trauma, in young patients with a gel vitreous body, causes sudden vitreomacular traction followed by forceful separation of them, leading to the formation of the macular holes.[26] Myopia is another cause leading to abnormal vitreoretinal physics, which causes the macular holes in a setting of retinal thinning in the center.[27] Other pathologies of macula or retina associated with macular hole formation are usually related to vitreoretinal interface abnormality.
Histopathology
SD-OCT has revolutionized the detailed architectural view of the macular hole. Typically, a true operculum (containing neuroretinal tissue) is not seen in a macular hole, compared to operculated holes in peripheral retinal degenerations that have a portion of avulsed retinal tissue (operculum) floating in the vitreous. However, two different types of opercula have been described in stage 3 full-thickness macular hole.[28]
- Pseudo-opercula (61%) - contains only glial tissue (Müller cells and fibrous astrocytes). Anatomical closure rates were better compared to true opercula, but visual outcomes were similar once the macular hole closed, though chances of a final vision of at least 20/40 were higher in the pseudo-opercula group.[28]
- True opercula (39%) - contains both glial tissue and neural tissue (cones) due to avulsion of neuroretinal tissue from full-thickness foveal tear. This also suggests that direct foveal traction has a role in the pathogenesis of macular holes.
Posterior cortical vitreoschisis occurs before macular hole formation, which leaves a hypocellular vitreous attached to the macula. Very minimum cellular proliferation usually takes place before the onset of a macular hole with minimum hypertrophy and hyperplasia of the retinal pigment epithelium.[29] The pseudo-operculum over the macular hole consists of vitreous condensation without neurosensory retinal components.
We often get the epiretinal membrane attached to the vitreoretinal interface.[30] Cystoid changes around the macular hole at any stage are well known due to preexisting vitreofoveal traction. This cystoid change is mainly due to the lamellar separation of retinal layers caused by vitreous traction.[31]
The loss of photoreceptors in the area of hole formation is another characteristic feature.[32]
History and Physical
An idiopathic macular hole is a disease of elderly individuals, mostly in their 6th to 7th decade of life, and females are more affected. The painless, gradual diminution of central vision with or without distorted vision is the main complaint. Initially, during the early stage of development of macular hole, vision may be mildly affected, but as it progresses visual problem increases.
Visual acuity reduction varies mildly in partial-thickness (PT) to markedly in a full-thickness macular hole (FTMH). Usually, slit-lamp biomicroscopy with +90D/+78D fundus viewing lenses confirms the diagnosis of FTMH as a red central spot.
Two different easy and simple examinations by slit lamp and laser-aiming beam can easily distinguish between a full-thickness macular hole and pseudo hole. After focussing a thin slit-lamp beam on the fovea with a fundus viewing lens, if the patient is asked to describe the thin slit, typically the patient describes a discontinuity or break in the midline i.e., Watzke-Allen test positive suggestive of full-thickness macular hole. Patients with a pseudo hole may describe as thinning of the slit in the center, or a bend at the center with no discontinuity. Varying sized laser-aiming beam (50 micrometers, 200 micrometers) if projected on the center of the macula in cases of a pseudo hole, can be seen but can not be detected by the patients with macular hole.[33]
Associated features may be evident in the form of epiretinal membrane i.e., perilesional puckering, involutional thinning, cystoid changes, complete posterior vitreous detachment (PVD), retinal pigment epithelium changes, pseudo-operculum, vitreomacular traction (VMT), and others. Depending on the stage of development, a yellowish cuff of fluid may be visible with yellowish dots in the center. The fellow eye must be examined for the presence of similar changes. PVD, if present, carries a lower risk of development of macular hole, if not present already.
Evaluation
Clinical examination is the mainstay of diagnosis of macular hole (MH). Associated features are also evident usually in the clinical examination. But as it is a disease of old age, associated cataract or other media opacities make it harder for the surgeon sometimes. OCT is the gold standard in the diagnosis, management, and follow-up in MH. It gives the three-dimensional cross-sectional picture of the hole and associated abnormality i.e., epiretinal membrane (ERM) or VMT mainly. It helps to prognosticate pre-operatively and follow up postoperatively. Different MH indices are now calculated to define the prognosis of the case[34] apart from the other prognostic factors in OCT.
Fundus fluorescence angiography shows central round hyperfluorescence due to a window defect. It may be done if associated with PCV, RAM, DR. Only macular hole does not usually need evaluation with angiography unless there are other factors including unexplained severe vision loss and others.
A method to confirm the macular hole and the pseudo hole is macular microperimetry using a scanning laser ophthalmoscope where functional follow-up can also be done.[35] Amsler grid test is another option, but it lacks specificity.[33][36]
Treatment / Management
As the diseased posterior cortical vitreous and abnormal vitreoretinal surface interaction are the main culprits, vitrectomy was tried as a treatment option after 1989 by Kelly and Wendel.[37] The surgical options are revolutionized with different developed instruments and drugs, vitreous substitutes, internal limiting membrane (ILM) and epiretinal membrane (ERM) staining dyes, and use of scaffold material to fill the gap in the macular hole (MH).
Stage 1 MH: It can be observed as chances of spontaneous resolution is near 50%. Surgical intervention has a negligible role in prevention in macular hole formation and it has its own complications whereas it has a high anatomical success rate if done in stage 2. In the case of significantly reduced visual acuity, the patient is symptomatic in stage 1 and persists for a long duration, surgery is usually advised.
Stage 2/3 MH: Spontaneous closure of FTMH may take place in 4 to 11.5% of cases.[38]
Usually, local anesthesia is preferred for surgery unless contraindicated otherwise. Pars-plana vitrectomy (PPV) is the mainstay of surgery following which the subsequent steps are performed. A standard 20/23/25/27-gauge core vitrectomy is performed initially. The posterior cortical vitreous is separated from the optic disc by using suction mode disabling cutter or by an extrusion cannula or bent needle which is a very important step. As posterior cortical vitreous is seen only after elevating it from the underlying retina triamcinolone acetonide assistance may be needed. Ocriplasmin, a serine protease, can also be used to separate vitreoretinal adhesion or to induce posterior vitreous detachment (PVD).[39] Induction and completion of PVD either surgically or by the pharmacologic agent are mandatory to remove all vitreoretinal interactions which can complicate the postoperative outcomes. After completing the vitrectomy of the cortical vitreous up to the equator, the peripheral vitreous is trimmed. The majority of MH cases no longer have any vertical vitreomacular traction because the posterior cortical vitreous is already separated from the macula. Specifically, vitreous around the ports should be removed meticulously to prevent traction from the insertion of the instruments and resulting retinal break or dialysis. Peeling of the internal liming membrane (ILM) is an important step, which has improved the rates of closure of macular holes.[40]
However, the induction of PVD remains the most crucial step in the management of stage 2 or 3 macular holes.
Only pharmacologic separation of vitreous from the retina by ocriplasmin intravitreal injection in a dose of 0.125 mg in 0.1 ml, is another way of treatment for a macular hole with a success rate of 40.6 - 67%.[41][42] Phakic eyes of less than 65-year-old patients with no ocular comorbidity, less than 250-micron diameter holes, vitreomacular adhesion of less than 1500 micron are most suitable for pharmacologic vitreolysis.[43]
Stage 4 MH: Apart from the induction of PVD all the steps are the same because in stage 4 MH PVD has already taken place.
In order to peel the ILM, to enhance visualization, and to have a precise less-traumatic surgery, ILM is stained using vital dyes i.e. indocyanine green (ICG 0.05%), trypan blue (TB 0.15%) (+/-) 10% glucose, and brilliant blue G (BBG 0.025%). Among them, ICG and BBG have selective affinity for ILM whereas TB stains ERM nicely than ILM. ICG has a contact period of 30 seconds and a concentration-dependent, osmolarity-dependent toxic effect on the retina along with light-induced damage that may take place when ICG is used.[44][45]
TB has a contact period of two minutes with very minimum toxicity. TB is used to stain ERM. BBG is the best alternative to ICG with a brief contact period, a good safety profile, and better visual outcomes.[46] In the presence of ERM, before removing it if ILM staining dye is given, ERM remains unstained on the stained ILM designated as 'negative' staining of ERM.
ILM peeling[40] is done to enhance the closure rate of MH and to prevent further reopening. It was noted that ILM removed from the cases of full-thickness macular hole contained Müller cell processes and myofibroblasts.[40] So to remove any sort of tractional contractile force on the macula, ILM peeling is very important.[40]
ILM is peeled after a pinch and tear an ILM flap with end-gripping forceps in a circular fashion not damaging the optic nerve fiber creating a "maculorrhexis" keeping fovea in the center. A diamond-dusted scrapper can also be used to elevate the flap but the risk of injury to the retina may be there. After successful peeling of ILM, it is removed and vitreous substitutes are injected to produce the tamponade effect. Recent advancement in this context is the inverted ILM flap technique, first described by Michalewska and colleagues[47][48] where the parafoveal ILM is not removed after peeling. It is folded inverted inside the FTMH to have the scaffold action.
After successful ILM peeling, intraocular gas tamponade by a fluid-air exchange which is usually followed by injection of isoexpansile gas enhances the chances of anatomical closure in the presence of proper head positioning.[49]
Different gases have been used including perfluoropropane (C3F8), perfluoroethane (C2F6), sulfur hexafluoride(SF6), air, and their combinations. The duration of tamponade in an operated eye and the presence of break/retinal detachment are important determinants for the choice of gas. Gases work by dehydrating the MH edge and preventing interaction with fluids inside.[50] Usually, it takes a week to close the hole. A large 17% C2F6–air mixture bubble after an extended vitrectomy can cover the macula even after a week following surgery in an upright position.[51]
Initial postoperative days are most crucial for the closure of a macular hole. Longer tamponade may not be necessary for the closure of a macular hole.[52]
A three-day tamponade in small MH is considered adequate and to have this effect 20% SF6-air mixture bubble or an air bubble alone is considered sufficient.[53] Silicone oil can be given where the patient is unable to maintain their head position or needs air traveling or in cases of failed previous surgery.
Head positioning is another important postoperative factor. It is considered that gas is needed to isolate the MH area from fluid interaction in the vitreous cavity in this period. If any mixture or gas can form a large bubble so that it would remain in contact with the large MH area for one week even in an upright position, then any head position can be chosen by the patient except supine.[49]
Differential Diagnosis
Recent advancement in OCT has made it simpler to differentiate a macular hole from other similar types of smaller diameter macular pathologies of old age. From history and physical examination, round central small reddish lesions with dimness of central vision, probable differential diagnosis include:
- Epiretinal membrane (ERM) with a macular pseudo hole
- Central foveal dot hemorrhage
- Lamellar (aborted) macular hole
- Vitreomacular traction syndrome (VMTS)
- Foveal drusen
- Central areolar pigment epitheliopathy
- Solar retinopathy
- Small choroidal neovascular membrane involving center
- Small central serous chorioretinopathy involving center
- Cystoid macular edema (CME)
Staging
There are two different staging systems of macular holes i.e., clinical staging and staging based on OCT findings. Gass has clinically described the stages of macular holes in the late '90s, but it is still accepted widely.[54]
- Stage 1 MH/impending MH: Loss of foveal depression. Stage 1A: detachment of foveola and yellow-colored spots in the center. Stage 1B foveolar detachment and yellow-colored ring surrounding detachment. Nearly 50% of Stage 1 MH undergo spontaneous resolution after vitreoretinal separation.
- Stage 2 MH: full-thickness neurosensory retinal defect in fovea but less than 400 micrometers in size. Sometimes the attached posterior cortical vitreous is evident on OCT. Almost 100% of this stage progress into stage 3.
- Stage 3 MH: full-thickness neurosensory retinal defect in fovea but greater than 400 micrometers in size. They are sometimes associated with a grayish macular rim, which indicates a cuff of subretinal fluid. Usually, PVD is started in this stage with or without operculum (but complete PVD or separation of the posterior vitreous face from the optic disc is absent), and 100% will go into stage 4.
- Stage 4 MH: stage 3 MH with complete PVD indicated by Weiss ring.
Based on OCT findings in the international vitreomacular traction study (IVTS) staging of vitreomacular adhesion (VMA), vitreomacular traction (VMT), MH has been described.[55]
- Vitreomacular adhesion (VMA): Absence of distortion of foveal contour
- Focal: Attached posterior hyaloid and retinal interface 1500 micrometer or less
- Broad: Greater than1500 micrometers
- Vitreomacular traction (VMT): Presence of distortion of foveal contour or structural changes present in intraretinal layers without a full-thickness macular hole
- Focal: Attached posterior hyaloid and retinal interface 1500 or fewer micrometers
- Broad: greater than 1500 micrometers.
Full-thickness macular hole (FTMH): Full-thickness neurosensory retinal defect from the internal limiting membrane to the retinal pigment epithelium.
- Factor 1: Size - horizontal diameter at narrowest point: small (less than or equal to 250 micrometers), medium (250 to 400 micrometer), large (greater than 400 micrometers)
- Factor 2: Cause - primary or secondary
- Factor 3: Presence/absence of VMT
Prognosis
Preoperative Prognostic Factors
Different OCT indices have revolutionized the assessment of the prognostic factors in macular holes (MHs).
Good Preoperative Prognostic Factors
- An idiopathic macular hole has a better prognosis than a traumatic macular hole which has not undergone spontaneous resolution and myopic MH.
- Less duration of symptoms
- Good preoperative vision
- Stage of the MH ( earlier the stage, better the prognosis)
- Size of MH (smaller the size, better the prognosis)
- OCT indices: Few OCT indices are calculated preoperatively to grade the prognosis.
Hole Forming Factor (HFF)
It is the ratio of the right and left arm length (measured to the minimum diameter of the hole from the base) to the base diameter of the MH. HFF greater than 0.9 carries a good prognosis, and less than 0.5 carries a poor prognosis for anatomical closure.[56]
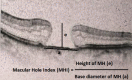
Macular Hole Index (MHI)
It indicates the ratio of MH height to MH base diameter. MHI greater than 0.5 carries a better prognosis.[57]
Diameter Hole Index (DHI)
It is the ratio of the minimum diameter of the hole to the diameter of the hole base. It signifies tangential traction. DHI less than 0.5 carries a good prognosis.[58]
Tractional Hole Index (THI)
It indicates the ratio of maximum MH height to the minimum diameter of MH. It signifies vertical traction or retinal hydration.[34] THI greater than 1.41 indicates a better prognosis.[59]
Cystic changes in retinal layers and subretinal fluid carry a good prognosis.[60]
Good Intraoperative Prognostic Factors
- Micro-incision vitrectomy surgery (MIVS)[61]
- ILM staining with BBG, rather than with ICG[62]
- Intraoperative tamponade agents: C3F8 is better than SF6, though this is controversial[63]
Good Postoperative Prognostic Factors
- Head positioning: Facedown positioning in larger (greater than 400 micrometers) macular holes is effective to close the hole[49]
- [49]The integrity of the inner segment: outer segment junction (IS: OS junction) or ellipsoid zone in OCT
- Type of closure: Type 1, i.e., without foveal neurosensory retinal (NSR) defect, carries a good prognosis, while type 2, i.e., with foveal NSR defect, carries a bad prognosis.[64]
Complications
Complications of macular hole (MH) surgery include complications of general intraocular surgery, pars plana vitrectomy (PPV), and other surgical steps i.e., ILM staining and peeling.
Complications of intraocular surgery, in general, include endophthalmitis, sympathetic ophthalmia, intraocular hemorrhage, glaucoma, hypotony, and recurrent corneal erosion.
Complications of pars plana vitrectomy (PPV): nuclear sclerotic cataract (most common), intraoperative cataract, intraoperative/postoperative retinal break (RB), retinal detachment (RD), and vitreous hemorrhage (VH), uveitis, anterior segment neovascularization, primary open-angle glaucoma.
Retinal Detachment (RD)
The incidence of RD after MH surgery is decreasing from 14% to 2% gradually.[65][66] The decrease is most probably due to the more energetic searching of intraoperative breaks and improved instrumentation.[67]
Complications of ILM Staining and Peeling
Among the dyes used to stain ILM, ICG causes concentration and osmolarity-dependent retinal pigment epithelium and ganglion cell toxicity, which is minimum with BBG. Another complication of ILM peeling is corrugations or irregularities in the retinal surface known as dissociated optic nerve fiber layer (DONFL). The incidence of DONFL was similar when cleavage plain was created with a diamond-dusted scraper or with forceps.[68]
DONFL causes ganglion cell damage resulting in microperimetry changes.[69][70]
Visual Field Defects
Postoperative inferotemporal visual field defect is not very uncommon.[71] Though advanced surgical techniques with vitreous and ILM staining procedures reduce its occurrence, causing minimum retinal trauma. The proposed hypothesis behind the field loss is the tractional force on the retina during the removal of the posterior cortical vitreous and dehydrated state of the nerve fiber layer opposite to the infusion port.[72][73]
Reopening of MH: Reopening of MH is not very common now after incorporating ILM peeling as a part of the surgery. Before the ILM peeling era, its incidence was 5% to 7%, then gradually decreasing to 0 to 2%.[74][75] If already peeled in the previous surgery, tangential traction to be ruled out by existing ILM. The second surgery must incorporate the release of existing traction or relaxing retinotomy. If no such traction is present, the only fluid-gas exchange can be done.[76][77][78] This reopening is more common in large MH, myopic MH, or where MH is associated with other pathologies.
A prolonged postoperative prone position may lead to ulnar neuropathy, which is a non-ocular complication of macular hole surgery.[79]
Side Effects of Intravitreal Ocriplasmin Injection
There can be a reduction in visual acuity, reduction in peripheral vision, retinal arterial attenuation, zonular damage/subluxation, or dislocation of the lens, ellipsoid zone integrity loss with poor response in electroretinogram are not uncommon. Pupillary abnormalities may also be there along with other side effects of any intravitreal injection i.e., cataract, vitreous hemorrhage, retinal detachment.[80]
Postoperative and Rehabilitation Care
Postoperative head positioning is an important factor determining the outcome of macular hole (MH) surgery. The aim is to isolate the MH area from fluid interaction to maintain the dehydrated state with the help of the gas tamponade. Usually face down position is not needed for small holes, provided that the gas bubble is large enough to isolate the hole area properly.[52]
Post-operative facedown position may help to close the macular hole of less than 400 micrometer diameter significantly.[49]
The patient should be counseled regarding the adverse effect of air-traveling on expansile gases. In those cases, silicone oil can be given.
Deterrence and Patient Education
The patient should be appropriately counseled regarding the prognostic factors and the possibilities of the closure of the macular hole. Anatomical closure doesn't always confirm good functional outcomes. Eyes having gas tamponade should be counseled to avoid air-traveling. Also, as gas is used, there is a drastic visual decline in the immediate postoperative period, which improves with time according to the gas used.
Pearls and Other Issues
Ocriplasmin-assisted vitreoretinal separation is becoming a significant part of the surgery to avoid retinal trauma with instruments used to induce PVD.[81] Inverted ILM flap or amniotic membrane in recurrent or reopened MH to cover the gap in MH is upcoming advancement in the field.[48][82][83] Autologous blood and autologous platelet concentrate are being used to enrich the MH area with growth factors so that effective closure will take place.[84][85] Mesenchymal stem cell graft in the macular hole surgery needs further trials to prove itself a crucial alternative.[86]
Enhancing Healthcare Team Outcomes
The technical assistance of SD-OCT has changed the surgical prognosis of MH. A technically skilled diagnostic team, along with efficient assistance during surgery, is crucial. Appropriate patient care during the rehabilitation period following surgery is of utmost importance. Effective collaboration between diagnostic, surgical, and nursing team is important to get better visual outcomes of the patient with early ambulation and recovery.

Figure
SD-OCT (macular scan) showing Vitreo-macular traction (VMT) syndrome Contributed by Soumyadeep Majumdar MS

Figure
SD-OCT (macular scan) of FTMH showing Hole Forming Factor (HFF) Contributed by Soumyadeep Majumdar, MS
Figure
SD-OCT (macular scan) showing calculation of Macular Hole Index (MHI) Contributed by Soumyadeep Majumdar, MS

Figure
SD-OCT (macular scan) showing calculation of Diameter Hole Index (DHI) Contributed by Soumyadeep Majumdar, MS

Figure
SD-OCT (macular scan) showing calculation of Tractional Hole Index (THI) Contributed by Soumyadeep Majumdar, MS
References
- 1.
- Gattoussi S, Buitendijk GHS, Peto T, Leung I, Schmitz-Valckenberg S, Oishi A, Wolf S, Deák G, Delcourt C, Klaver CCW, Korobelnik JF., European Eye Epidemiology (E3) consortium. The European Eye Epidemiology spectral-domain optical coherence tomography classification of macular diseases for epidemiological studies. Acta Ophthalmol. 2019 Jun;97(4):364-371. [PubMed: 30242982]
- 2.
- Azzolini C. Macular Hole: From Diagnosis to Therapy. J Ophthalmol. 2020;2020:1473763. [PMC free article: PMC7125507] [PubMed: 32280514]
- 3.
- Stein GE, Jung JJ, Bodine S, Trokel SL, Chang S. Vitrectomy for macular hole following Nd:YAG laser injury. Taiwan J Ophthalmol. 2016 Oct-Dec;6(4):195-198. [PMC free article: PMC5525626] [PubMed: 29018741]
- 4.
- Pillai GS, Varkey R, Unnikrishnan UG, Radhakrishnan N. Incidence and risk factors for intraocular pressure rise after transconjunctival vitrectomy. Indian J Ophthalmol. 2020 May;68(5):812-817. [PMC free article: PMC7350492] [PubMed: 32317451]
- 5.
- Chino M, Yoshikawa Y, Kanno J, Nagashima T, Sakaki Y, Katsumoto T, Shibuya M, Shoji T, Makita J, Shinoda K. Development and spontaneous closure of a secondary macular hole associated with submacular hemorrhage due to polypoidal choroidal vasculopathy: a case report. BMC Ophthalmol. 2020 Mar 17;20(1):108. [PMC free article: PMC7079491] [PubMed: 32183733]
- 6.
- Peart S, Ramsay A, Khan QA, Leong T, Gordon-Bennett P. Large, Spontaneous Macular Hole with Posterior Pole Detachment in a Patient with Best Vitelliform Macular Dystrophy. Case Rep Ophthalmol. 2019 May-Aug;10(2):221-226. [PMC free article: PMC6760364] [PubMed: 31692623]
- 7.
- Tsuiki E, Kusano M, Kitaoka T. Complication associated with intravitreal injection of tissue plasminogen activator for treatment of submacular hemorrhage due to rupture of retinal arterial macroaneurysm. Am J Ophthalmol Case Rep. 2019 Dec;16:100556. [PMC free article: PMC6804720] [PubMed: 31650084]
- 8.
- Levison AL, Schachat AP. Macular hole from a central retinal artery occlusion. JAMA Ophthalmol. 2014 Dec;132(12):1493. [PubMed: 25275337]
- 9.
- Fragiotta S, Rossi T, Carnevale C, Cutini A, Tricarico S, Casillo L, Scuderi G, Vingolo EM. Vitreo-macular interface disorders in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2019 Oct;257(10):2137-2146. [PubMed: 31324966]
- 10.
- Raimundo M, Fonseca C, Silva R, Figueira J. Bilateral giant macular holes: A rare manifestation of Alport syndrome. Eur J Ophthalmol. 2019 Jan;29(1):NP13-NP16. [PubMed: 29873249]
- 11.
- Xie ZG, Yu SQ, Chen X, Zhu J, Chen F. Macular hole secondary to Valsalva retinopathy after doing push-up exercise. BMC Ophthalmol. 2014 Aug 12;14:98. [PMC free article: PMC4136644] [PubMed: 25117955]
- 12.
- Rizzo S, Mucciolo DP, Bacherini D, Murro V, Vannozzi L, Virgili G, Bani D, Sodi A. Macular hole in Stargardt disease: Clinical and ultra-structural observation. Ophthalmic Genet. 2017 Sep-Oct;38(5):486-489. [PubMed: 28121212]
- 13.
- Li Y, Li Z, Xu C, Liu Y, Kang X, Wu J. Autologous neurosensory retinal transplantation for recurrent macular hole retinal detachment in highly myopic eyes. Acta Ophthalmol. 2020 Dec;98(8):e983-e990. [PubMed: 32323479]
- 14.
- Liao DY, Liu JH, Zheng YP, Shiu HW, Wang JM, Chao HM. OCT proves that vitreomacular adhesion is significantly more likely to develop vision-threatening retinal complications than vitreomacular separation. BMC Ophthalmol. 2020 Apr 22;20(1):163. [PMC free article: PMC7178608] [PubMed: 32321473]
- 15.
- Gao M, Liu K, Lin Q, Liu H. Management Modalities for Traumatic Macular Hole: A Systematic Review and Single-Arm Meta-Analysis. Curr Eye Res. 2017 Feb;42(2):287-296. [PubMed: 27420902]
- 16.
- Chen HJ, Jin Y, Shen LJ, Wang Y, Li ZY, Fang XY, Wang ZL, Huang XD, Wang ZJ, Ma ZZ. Traumatic macular hole study: a multicenter comparative study between immediate vitrectomy and six-month observation for spontaneous closure. Ann Transl Med. 2019 Dec;7(23):726. [PMC free article: PMC6989969] [PubMed: 32042742]
- 17.
- Lewis ML, Cohen SM, Smiddy WE, Gass JD. Bilaterality of idiopathic macular holes. Graefes Arch Clin Exp Ophthalmol. 1996 Apr;234(4):241-5. [PubMed: 8964529]
- 18.
- Trempe CL, Weiter JJ, Furukawa H. Fellow eyes in cases of macular hole. Biomicroscopic study of the vitreous. Arch Ophthalmol. 1986 Jan;104(1):93-5. [PubMed: 3942551]
- 19.
- la Cour M, Friis J. Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmol Scand. 2002 Dec;80(6):579-87. [PubMed: 12485276]
- 20.
- McCannel CA, Ensminger JL, Diehl NN, Hodge DN. Population-based incidence of macular holes. Ophthalmology. 2009 Jul;116(7):1366-9. [PMC free article: PMC2867090] [PubMed: 19576500]
- 21.
- Ali FS, Stein JD, Blachley TS, Ackley S, Stewart JM. Incidence of and Risk Factors for Developing Idiopathic Macular Hole Among a Diverse Group of Patients Throughout the United States. JAMA Ophthalmol. 2017 Apr 01;135(4):299-305. [PMC free article: PMC5470400] [PubMed: 28208188]
- 22.
- Risk factors for idiopathic macular holes. The Eye Disease Case-Control Study Group. Am J Ophthalmol. 1994 Dec 15;118(6):754-61. [PubMed: 7977602]
- 23.
- Morgan CM, Schatz H. Involutional macular thinning. A pre-macular hole condition. Ophthalmology. 1986 Feb;93(2):153-61. [PubMed: 3951821]
- 24.
- Ozawa Y, Shinoda H, Nagai N, Tsubota K. Dynamic changes in neural retinal images during the development of a lamellar macular hole: A case report. Medicine (Baltimore). 2019 Dec;98(49):e18297. [PMC free article: PMC6919539] [PubMed: 31804376]
- 25.
- Green WR. The macular hole: histopathologic studies. Arch Ophthalmol. 2006 Mar;124(3):317-21. [PubMed: 16534050]
- 26.
- Liu J, Peng J, Zhang Q, Ma M, Zhang H, Zhao P. Etiologies, Characteristics, and Management of Pediatric Macular Hole. Am J Ophthalmol. 2020 Feb;210:174-183. [PubMed: 31560879]
- 27.
- Xin W, Cai X, Xiao Y, Ji L, Gu Y, Lv W, Jiang J. Surgical treatment for type II macular hole retinal detachment in pathologic myopia. Medicine (Baltimore). 2020 Apr;99(17):e19531. [PMC free article: PMC7220656] [PubMed: 32332602]
- 28.
- Ezra E, Munro PM, Charteris DG, Aylward WG, Luthert PJ, Gregor ZJ. Macular hole opercula. Ultrastructural features and clinicopathological correlation. Arch Ophthalmol. 1997 Nov;115(11):1381-7. [PubMed: 9366667]
- 29.
- Sebag J. [The vitreoretinal interface and its role in the pathogenesis of vitreomaculopathies]. Ophthalmologe. 2015 Jan;112(1):10-9. [PubMed: 25681055]
- 30.
- Quinn NB, Steel DH, Chakravarthy U, Peto T, Hamill B, Muldrew A, Graham K, Elliott D, Hennessy R, Cruise S, McGuinness B, Young IS, Kee F, Hogg RE. Assessment of the Vitreomacular Interface Using High-Resolution OCT in a Population-Based Cohort Study of Older Adults. Ophthalmol Retina. 2020 Aug;4(8):801-813. [PubMed: 32335034]
- 31.
- Folk JC, Boldt HC, Keenum DG. Foveal cysts: a premacular hole condition associated with vitreous traction. Arch Ophthalmol. 1998 Sep;116(9):1177-83. [PubMed: 9747675]
- 32.
- Cicinelli MV, Marchese A, Bandello F, Coppola M. Inner Retinal Layer and Outer Retinal Layer Findings after Macular Hole Surgery Assessed by means of Optical Coherence Tomography. J Ophthalmol. 2019;2019:3821479. [PMC free article: PMC6466935] [PubMed: 31061725]
- 33.
- Saxena S, Holekamp NM, Kumar A. Diagnosis and management of idiopathic macular holes. Indian J Ophthalmol. 1998 Dec;46(4):185-93. [PubMed: 10218300]
- 34.
- Venkatesh R, Mohan A, Sinha S, Aseem A, Yadav NK. Newer indices for predicting macular hole closure in idiopathic macular holes: A retrospective, comparative study. Indian J Ophthalmol. 2019 Nov;67(11):1857-1862. [PMC free article: PMC6836585] [PubMed: 31638049]
- 35.
- Sjaarda RN, Frank DA, Glaser BM, Thompson JT, Murphy RP. Resolution of an absolute scotoma and improvement of relative scotomata after successful macular hole surgery. Am J Ophthalmol. 1993 Aug 15;116(2):129-39. [PubMed: 8352296]
- 36.
- Tripathy K, Salini B. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 25, 2023. Amsler Grid. [PubMed: 30844168]
- 37.
- Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991 May;109(5):654-9. [PubMed: 2025167]
- 38.
- Liang X, Liu W. Characteristics and Risk Factors for Spontaneous Closure of Idiopathic Full-Thickness Macular Hole. J Ophthalmol. 2019;2019:4793764. [PMC free article: PMC6436358] [PubMed: 31001430]
- 39.
- Khan MA, Haller JA. Ocriplasmin for Treatment of Vitreomacular Traction: An Update. Ophthalmol Ther. 2016 Dec;5(2):147-159. [PMC free article: PMC5125123] [PubMed: 27619226]
- 40.
- Eckardt C, Eckardt U, Groos S, Luciano L, Reale E. [Removal of the internal limiting membrane in macular holes. Clinical and morphological findings]. Ophthalmologe. 1997 Aug;94(8):545-51. [PubMed: 9376691]
- 41.
- Muqit MMK, Hamilton R, Ho J, Tucker S, Buck H. Intravitreal ocriplasmin for the treatment of vitreomacular traction and macular hole- A study of efficacy and safety based on NICE guidance. PLoS One. 2018;13(5):e0197072. [PMC free article: PMC5955569] [PubMed: 29768451]
- 42.
- Juncal VR, Chow DR, Vilà N, Kapusta MA, Williams RG, Kherani A, Berger AR. Ocriplasmin versus vitrectomy for the treatment of macular holes. Can J Ophthalmol. 2018 Oct;53(5):441-446. [PubMed: 30340707]
- 43.
- Prospero Ponce CM, Stevenson W, Gelman R, Agarwal DR, Christoforidis JB. Ocriplasmin: who is the best candidate? Clin Ophthalmol. 2016;10:485-95. [PMC free article: PMC4803238] [PubMed: 27051270]
- 44.
- Rodrigues EB, Meyer CH. Meta-analysis of chromovitrectomy with indocyanine green in macular hole surgery. Ophthalmologica. 2008;222(2):123-9. [PubMed: 18303234]
- 45.
- Stalmans P, Van Aken EH, Veckeneer M, Feron EJ, Stalmans I. Toxic effect of indocyanine green on retinal pigment epithelium related to osmotic effects of the solvent. Am J Ophthalmol. 2002 Aug;134(2):282-5. [PubMed: 12140045]
- 46.
- Shukla D, Kalliath J, Neelakantan N, Naresh KB, Ramasamy K. A comparison of brilliant blue G, trypan blue, and indocyanine green dyes to assist internal limiting membrane peeling during macular hole surgery. Retina. 2011 Nov;31(10):2021-5. [PubMed: 21685824]
- 47.
- Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010 Oct;117(10):2018-25. [PubMed: 20541263]
- 48.
- Michalewska Z, Nawrocki J. Vitrectomy with the inverted internal limiting membrane flap technique in eyes with full-thickness macular hole and dry age-related macular degeneration. Eur J Ophthalmol. 2021 May;31(3):1320-1325. [PubMed: 32345051]
- 49.
- Ye T, Yu JG, Liao L, Liu L, Xia T, Yang LL. Macular hole surgery recovery with and without face-down posturing: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2019 Dec 21;19(1):265. [PMC free article: PMC6925505] [PubMed: 31864333]
- 50.
- Berger JW, Brucker AJ. The magnitude of the bubble buoyant pressure: implications for macular hole surgery. Retina. 1998;18(1):84-6; author reply 86-8. [PubMed: 9502292]
- 51.
- Paques M, Massin P, Blain P, Duquesnoy AS, Gaudric A. Long-term incidence of reopening of macular holes. Ophthalmology. 2000 Apr;107(4):760-5; discussion 766. [PubMed: 10768340]
- 52.
- Yamashita T, Sakamoto T, Yamashita T, Sonoda S, Yamakiri K, Otsuka H, Hisatomi T, Imaki H, Ishibashi T, Dugel PU. Individualized, spectral domain-optical coherence tomography-guided facedown posturing after macular hole surgery: minimizing treatment burden and maximizing outcome. Retina. 2014 Jul;34(7):1367-75. [PubMed: 24955569]
- 53.
- Wirbelauer C, Kolarov D, Just A. [Influence of Macular Hole Width on Visual Acuity, Endotamponade and Closure Rate]. Klin Monbl Augenheilkd. 2016 Dec;233(12):1362-1366. [PubMed: 27984839]
- 54.
- Gass JD. Idiopathic senile macular hole: its early stages and pathogenesis. 1988. Retina. 2003 Dec;23(6 Suppl):629-39. [PubMed: 15035400]
- 55.
- Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, Sadda SR, Sebag J, Spaide RF, Stalmans P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013 Dec;120(12):2611-2619. [PubMed: 24053995]
- 56.
- Ullrich S, Haritoglou C, Gass C, Schaumberger M, Ulbig MW, Kampik A. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002 Apr;86(4):390-3. [PMC free article: PMC1771090] [PubMed: 11914205]
- 57.
- Kusuhara S, Teraoka Escaño MF, Fujii S, Nakanishi Y, Tamura Y, Nagai A, Yamamoto H, Tsukahara Y, Negi A. Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am J Ophthalmol. 2004 Nov;138(5):709-16. [PubMed: 15531303]
- 58.
- Ruiz-Moreno JM, Staicu C, Piñero DP, Montero J, Lugo F, Amat P. Optical coherence tomography predictive factors for macular hole surgery outcome. Br J Ophthalmol. 2008 May;92(5):640-4. [PubMed: 18441174]
- 59.
- Dai YM, Shen J, Li JK, Jin XH, Li YM. [Optical coherence tomography predictive factors for idiopathic macular hole surgery outcome]. Zhonghua Yan Ke Za Zhi. 2013 Sep;49(9):807-11. [PubMed: 24330930]
- 60.
- Shahlaee A, Rahimy E, Hsu J, Gupta OP, Ho AC. Preoperative and postoperative features of macular holes on en face imaging and optical coherence tomography angiography. Am J Ophthalmol Case Rep. 2017 Apr;5:20-25. [PMC free article: PMC5758006] [PubMed: 29503940]
- 61.
- Chen GH, Tzekov R, Jiang FZ, Mao SH, Tong YH, Li WS. Iatrogenic retinal breaks and postoperative retinal detachments in microincision vitrectomy surgery compared with conventional 20-gauge vitrectomy: a meta-analysis. Eye (Lond). 2019 May;33(5):785-795. [PMC free article: PMC6707291] [PubMed: 30560911]
- 62.
- Li SS, You R, Li M, Guo XX, Zhao L, Wang YL, Chen X. Internal limiting membrane peeling with different dyes in the surgery of idiopathic macular hole: a systematic review of literature and network Meta-analysis. Int J Ophthalmol. 2019;12(12):1917-1928. [PMC free article: PMC6901880] [PubMed: 31850178]
- 63.
- Unsal E, Cubuk MO, Ciftci F. Preoperative prognostic factors for macular hole surgery: Which is better? Oman J Ophthalmol. 2019 Jan-Apr;12(1):20-24. [PMC free article: PMC6380157] [PubMed: 30787530]
- 64.
- Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol. 2003 Aug;87(8):1015-9. [PMC free article: PMC1771782] [PubMed: 12881347]
- 65.
- Park SS, Marcus DM, Duker JS, Pesavento RD, Topping TM, Frederick AR, D'Amico DJ. Posterior segment complications after vitrectomy for macular hole. Ophthalmology. 1995 May;102(5):775-81. [PubMed: 7777277]
- 66.
- Rizzo S, Belting C, Genovesi-Ebert F, di Bartolo E. Incidence of retinal detachment after small-incision, sutureless pars plana vitrectomy compared with conventional 20-gauge vitrectomy in macular hole and epiretinal membrane surgery. Retina. 2010 Jul-Aug;30(7):1065-71. [PubMed: 20616684]
- 67.
- Guillaubey A, Malvitte L, Lafontaine PO, Hubert I, Bron A, Berrod JP, Creuzot-Garcher C. Incidence of retinal detachment after macular surgery: a retrospective study of 634 cases. Br J Ophthalmol. 2007 Oct;91(10):1327-30. [PMC free article: PMC2001011] [PubMed: 17522152]
- 68.
- Runkle AP, Srivastava SK, Yuan A, Kaiser PK, Singh RP, Reese JL, Ehlers JP. FACTORS ASSOCIATED WITH DEVELOPMENT OF DISSOCIATED OPTIC NERVE FIBER LAYER APPEARANCE IN THE PIONEER INTRAOPERATIVE OPTICAL COHERENCE TOMOGRAPHY STUDY. Retina. 2018 Sep;38 Suppl 1(Suppl 1):S103-S109. [PMC free article: PMC6047929] [PubMed: 29346239]
- 69.
- Spaide RF. "Dissociated optic nerve fiber layer appearance" after internal limiting membrane removal is inner retinal dimpling. Retina. 2012 Oct;32(9):1719-26. [PubMed: 23007669]
- 70.
- Tadayoni R, Svorenova I, Erginay A, Gaudric A, Massin P. Decreased retinal sensitivity after internal limiting membrane peeling for macular hole surgery. Br J Ophthalmol. 2012 Dec;96(12):1513-6. [PMC free article: PMC3512349] [PubMed: 23077227]
- 71.
- Paques M, Massin P, Santiago PY, Spielmann AC, Gaudric A. Visual field loss after vitrectomy for full-thickness macular holes. Am J Ophthalmol. 1997 Jul;124(1):88-94. [PubMed: 9222237]
- 72.
- Ezra E, Arden GB, Riordan-Eva P, Aylward GW, Gregor ZJ. Visual field loss following vitrectomy for stage 2 and 3 macular holes. Br J Ophthalmol. 1996 Jun;80(6):519-25. [PMC free article: PMC505524] [PubMed: 8759262]
- 73.
- Welch JC. Dehydration injury as a possible cause of visual field defect after pars plana vitrectomy for macular hole. Am J Ophthalmol. 1997 Nov;124(5):698-9. [PubMed: 9372731]
- 74.
- Christmas NJ, Smiddy WE, Flynn HW. Reopening of macular holes after initially successful repair. Ophthalmology. 1998 Oct;105(10):1835-8. [PubMed: 9787352]
- 75.
- Rahimy E, McCannel CA. IMPACT OF INTERNAL LIMITING MEMBRANE PEELING ON MACULAR HOLE REOPENING: A Systematic Review and Meta-Analysis. Retina. 2016 Apr;36(4):679-87. [PubMed: 26441264]
- 76.
- Paques M, Massin P, Santiago PY, Spielmann AC, Le Gargasson JF, Gaudric A. Late reopening of successfully treated macular holes. Br J Ophthalmol. 1997 Aug;81(8):658-62. [PMC free article: PMC1722272] [PubMed: 9349153]
- 77.
- Knight D, Yu JJ, Adrean SD. RELAXING NASAL RETINOTOMY TECHNIQUE FOR CLOSURE OF A MACULAR HOLE THAT REOPENED AFTER PRIMARY VITRECTOMY. Retin Cases Brief Rep. 2021 Sep 01;15(5):611-614. [PubMed: 30865057]
- 78.
- Rao X, Wang NK, Chen YP, Hwang YS, Chuang LH, Liu IC, Chen KJ, Wu WC, Lai CC. Outcomes of outpatient fluid-gas exchange for open macular hole after vitrectomy. Am J Ophthalmol. 2013 Aug;156(2):326-333.e1. [PubMed: 23688710]
- 79.
- Holekamp NM, Meredith TA, Landers MB, Snyder WB, Thompson JT, Berman AJ, Williams S. Ulnar neuropathy as a complication of macular hole surgery. Arch Ophthalmol. 1999 Dec;117(12):1607-10. [PubMed: 10604664]
- 80.
- Fahim AT, Khan NW, Johnson MW. Acute panretinal structural and functional abnormalities after intravitreous ocriplasmin injection. JAMA Ophthalmol. 2014 Apr 01;132(4):484-6. [PubMed: 24577241]
- 81.
- Zandi S, Freiberg F, Vaclavik V, Pfister IB, Traine PG, Kaya C, Michels S, Garweg JG. Morphological Reconstitution and Persistent Changes After Intravitreal Ocriplasmin for Vitreomacular Traction and Macular Hole. J Ocul Pharmacol Ther. 2020 Mar;36(2):126-132. [PubMed: 31934816]
- 82.
- Florent C, Coupier L, Mérité PY, Meyer F, Guigou S. Human amniotic membrane plug technique for macular hole surgery: A case report. J Fr Ophtalmol. 2020 Apr;43(4):e151-e152. [PubMed: 32111503]
- 83.
- Caporossi T, Pacini B, De Angelis L, Barca F, Peiretti E, Rizzo S. HUMAN AMNIOTIC MEMBRANE TO CLOSE RECURRENT, HIGH MYOPIC MACULAR HOLES IN PATHOLOGIC MYOPIA WITH AXIAL LENGTH OF ≥30 mm. Retina. 2020 Oct;40(10):1946-1954. [PubMed: 31868775]
- 84.
- Lai CC, Chen YP, Wang NK, Chuang LH, Liu L, Chen KJ, Hwang YS, Wu WC, Chen TL. Vitrectomy with Internal Limiting Membrane Repositioning and Autologous Blood for Macular Hole Retinal Detachment in Highly Myopic Eyes. Ophthalmology. 2015 Sep;122(9):1889-98. [PubMed: 26143541]
- 85.
- Kapoor KG, Khan AN, Tieu BC, Khurshid GS. Revisiting autologous platelets as an adjuvant in macular hole repair: chronic macular holes without prone positioning. Ophthalmic Surg Lasers Imaging. 2012 Jul 01;43(4):291-5. [PubMed: 22589336]
- 86.
- Zhang X, Liu J, Yu B, Ma F, Ren X, Li X. Effects of mesenchymal stem cells and their exosomes on the healing of large and refractory macular holes. Graefes Arch Clin Exp Ophthalmol. 2018 Nov;256(11):2041-2052. [PubMed: 30167916]
Disclosure: Soumyadeep Majumdar declares no relevant financial relationships with ineligible companies.
Disclosure: Koushik Tripathy declares no relevant financial relationships with ineligible companies.
- Continuing Education Activity
- Introduction
- Etiology
- Epidemiology
- Pathophysiology
- Histopathology
- History and Physical
- Evaluation
- Treatment / Management
- Differential Diagnosis
- Staging
- Prognosis
- Complications
- Postoperative and Rehabilitation Care
- Deterrence and Patient Education
- Pearls and Other Issues
- Enhancing Healthcare Team Outcomes
- Review Questions
- References
- Stage 0 macular holes: observations by optical coherence tomography.[Ophthalmology. 2004]Stage 0 macular holes: observations by optical coherence tomography.Chan A, Duker JS, Schuman JS, Fujimoto JG. Ophthalmology. 2004 Nov; 111(11):2027-32.
- Foveal pseudocyst as the first step in macular hole formation: a prospective study by optical coherence tomography.[Ophthalmology. 2001]Foveal pseudocyst as the first step in macular hole formation: a prospective study by optical coherence tomography.Haouchine B, Massin P, Gaudric A. Ophthalmology. 2001 Jan; 108(1):15-22.
- Value of internal limiting membrane peeling in surgery for idiopathic macular hole and the correlation between function and retinal morphology.[Acta Ophthalmol. 2009]Value of internal limiting membrane peeling in surgery for idiopathic macular hole and the correlation between function and retinal morphology.Christensen UC. Acta Ophthalmol. 2009 Dec; 87 Thesis 2:1-23.
- Review Microstructural and hemodynamic changes in the fundus after pars plana vitrectomy for different vitreoretinal diseases.[Graefes Arch Clin Exp Ophthalm...]Review Microstructural and hemodynamic changes in the fundus after pars plana vitrectomy for different vitreoretinal diseases.Li D, Chen H, Huang S, Jia B, Lu L, Fu J. Graefes Arch Clin Exp Ophthalmol. 2023 Nov 20; . Epub 2023 Nov 20.
- Review Idiopathic Macular Hole: A Comprehensive Review of Its Pathogenesis and of Advanced Studies on Metamorphopsia.[J Ophthalmol. 2019]Review Idiopathic Macular Hole: A Comprehensive Review of Its Pathogenesis and of Advanced Studies on Metamorphopsia.Chen Q, Liu ZX. J Ophthalmol. 2019; 2019:7294952. Epub 2019 May 23.
- Macular Hole - StatPearlsMacular Hole - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
See more...