NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Garcinia cambogia is an herbal product derived from the fruit of the Malabar tamarind tree native to Southeastern Asia which is used as a food preservative and flavoring agent and has recently been used increasingly in herbal weight loss products. Weight loss products labeled as containing Garcinia cambogia been linked to the development of clinically apparent acute liver injury which can be severe and even fatal.
Background
Garcinia cambogia is an herbal product derived from fruit of the Malabar tamarind tree (also called Garcinia gummi-gutta) which is native to India, Nepal and Sri Lanka. The dried and smoked rind of the Garcinia cambogia fruit is commonly used as a food preservative and, because of its sharp, sour taste, as a flavoring agent and spice, especially in fish curries. The fruit rind has also been used in Ayurvedic medicine to treat gastrointestinal complaints and rheumatism. More recently, Garcinia cambogia has been purported to be an appetite suppressant effective in inducing weight loss. Chemical components of Garcinia extracts include xanthones, benzophenones, amino acids and organic acids, most importantly hydroxycitric acid (HCA) the suspected ingredient responsible for its antiinflammatory and appetite suppressant activity. Garcinia fruit is 10% to 30% HCA by weight, and extracts can contain between 20% and 60% of the tricarboxylic acid. HCA inhibits ATP citrate lyase an enzyme involved in fatty acid synthesis. Garcinia derivatives and HCA have been shown to cause appetite suppression and weight loss in rats, but the effects of these organic acids and Garcinia extracts have not been consistently found in human studies. Nevertheless, Garcinia cambogia is a frequent component of over-the-counter multiingredient herbal products and has been advertised as a weight loss product. Studies in rats and other animal models have suggested that Garcinia cambogia and HCA do not have significant toxicities, although testicular toxicity was found with high doses. In humans, Garcinia has been linked to rare reports of serotonin syndrome, rhabdomyolysis and hepatic toxicity, but the role of Garcinia as opposed to other components of the herbal products or mixtures typically used in humans has not been clearly defined.
Hepatotoxicity
There have been at least a dozen reports of Garcinia induced liver injury in individuals taking multiingredient dietary supplements (MIDS) that contain Garcinia cambogia. In some instances, other known or suspected hepatotoxins were present in the MIDS products (such as with Exilis, Herbalife and Hydroxycut products). More recently, cases of acute liver injury have been identified in persons taking a product labelled as having Garcinia cambogia alone. The frequency of hepatic adverse reactions to Garcinia cambogia is not known but is likely uncommon and in less than 1:10,000 persons. Patients typically present with fatigue, nausea, elevations in serum aminotransferase levels, and jaundice 1 to 4 weeks after starting the product, although the latency to onset has been longer (3 to 12 months) in some cases. The pattern of enzyme elevations is hepatocellular, and immune features are not common. Some cases have been severe, resulting in acute liver failure and either death or need for urgent liver transplantation. Recurrence upon reexposure has not been clearly shown.
Likelihood score: B (likely rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of hepatotoxicity from Garcinia cambogia is unclear. In vitro studies suggest that HCA may be toxic to the liver in high doses, but the rare instances of acute liver injury that occur with Garcinia cambogia suggest an idiosyncratic form of injury. The possibility of mislabeling or adulteration with hepatotoxic herbal products is always an issue in herbal related injury.
Outcome and Management
The severity of liver injury ranges from transient and moderate enzyme elevations to symptomatic acute hepatitis to acute liver failure. In most instances, the liver injury subsides within 1 to 3 months of discontinuing the herbal product, but if fulminant hepatitis develops, a liver transplant may be required. Rechallenge should be avoided.
Other Names: Gamboge, Brindleberry, Malabar tamarind. There are at least 400 species of Garcinia other than cambogia (gummi-gutta) which may or may not have similar antiinflammatory and appetite suppressant activity or similar concentrations of HCA. Indeed, mislabeling other Garcinia species as cambogia may account for some instances of unexpected toxicity.
Drug Class: Herbal and Dietary Supplements
CASE REPORT
Case 1. Garcinia associated acute hepatitis.
[Modified from Case 1 in: Crescioli G, Lombardi N, Bettiol A, Marconi E, Risaliti F, Bertoni M, Menniti Ippolito F, et al. Acute liver injury following Garcinia cambogia weight-loss supplementation: case series and literature review. Intern Emerg Med 2018 May 25. [Epub ahead of print] PubMed Citation]
A 61 year old woman developed abdominal pain, nausea, dark urine and jaundice approximately 6 weeks after starting a weight loss product called Super Ananas Slim, which was labelled as containing Garcinia cambogia. She had no history of liver disease, alcohol abuse or exposure to hepatotoxins or acetaminophen. Her other medical conditions included history of cholecystectomy, dyslipidemia and hypothyroidism and her only other medication was levothyroxine. Two weeks after onset of symptoms, laboratory tests showed a serum bilirubin of 22.5 mg/dL (direct 16.7 mg/dL), ALT 1629 U/L, AST 1121 U/L, alkaline phosphatase 150 U/L and INR 2.2. She tested negative for hepatitis A, B and C and for routine autoantibodies. Despite stopping the herbal product, jaundice and symptoms worsened and persisted for several weeks, but ultimately resolved although serum aminotransferase levels remained mildly elevated 5 months later.
Key Points
| Medication: | Garcinia cambogia |
|---|---|
| Pattern: | Hepatocellular (R=30) |
| Severity: | 4+ (jaundice and prolonged INR) |
| Latency: | 6 weeks |
| Recovery: | Slow, incomplete at 5 months |
| Other medications: | Levothyroxine |
Laboratory Values
Comment
This patient developed a moderately severe acute hepatitis-like syndrome within 6 weeks of starting a multiingredient weight loss product called “Ananas Slim” which contained Garcinia cambogia as the primary ingredient, but it had other components including Ananas comosus (pineapple) and Ilex paraguariensis (Yerba mate), neither of which have been implicated in hepatotoxicity or other adverse effects. Similar to other reports of Garcinia associated liver injury, the latency to onset was rather brief (6 weeks) and the clinical features were an acute hepatocellular injury with significant jaundice and hepatic dysfunction. The injury was fairly severe and liver test abnormalities were still present 5 months later.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Garcinia Cambogia – Generic
DRUG CLASS
Herbal and Dietary Supplements
COMPLETE LABELING
Fact Sheet at National Center for Complementary and Integrative Health
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Garcinia Cambogia | 6205-14-7 | C6-H8-O8 |
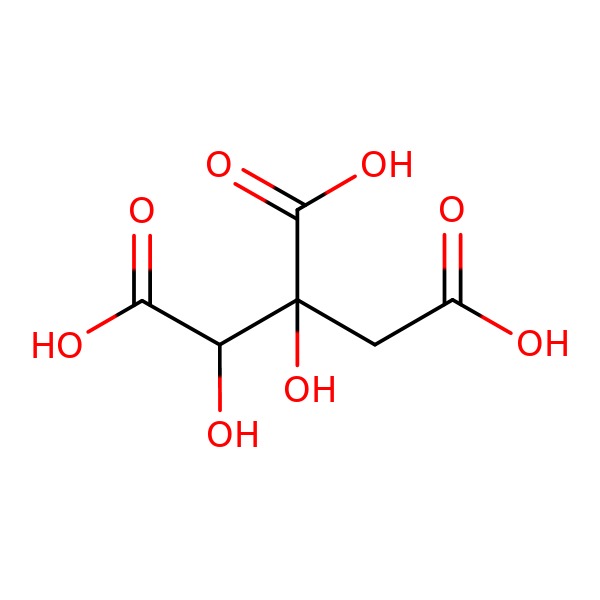 |
ANNOTATED BIBLIOGRAPHY
References updated: 13 February 2019
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott,1999: pp. 731-4.(Expert review of hepatotoxicity published in 1999; several herbal medications linked to liver injury are discussed, but not Garcinia).
- Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbals and dietary supplements. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 631-58.(Review of hepatotoxicity of herbal and dietary supplements; does not mention Garcinia cambogia).
- Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nunez C. Garcinia cambogia(hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. JAMA 1998; 280: 1596-600. [PubMed: 9820262](Among 135 obese adults treated with HCA [1500 mg daily] or placebo for 12 weeks, weight loss was similar in the two groups [3.2 vs 4.1 kg] and adverse event rates were similar, and none led to early discontinuations; no mention of ALT testing or hepatotoxicity).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, including 7 [5%] for herbal medications, but none were attributed to Garcinia).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology 2005; 129: 512-21. [PubMed: 16083708](Reports of drug induced liver injury to a Spanish network found 570 cases, herbal medications accounted for 9 cases, but none were attributed to Garcinia).
- Stevens T, Qadri A, Zein NN. Two patients with acute liver injury associated with use of the herbal weight-loss supplement hydroxycut. Ann Intern Med 2005; 142: 477-8. [PubMed: 15767636](27 and 30-year old men developed jaundice 2 and 5 weeks after starting a Hydroxycut product [bilirubin 7.8 and 7.8 mg/dL, ALT 3131 and 45 U/L, Alk P 171 and 530 U/L], resolving in 5 weeks).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 9% of cases were attributed to herbal medications, but Garcinia cambogia was not specifically mentioned).
- García-Cortés M, Borraz Y, Lucena MI, Peláez G, Salmerón J, Diago M, Martínez-Sierra MC, et al. [Liver injury induced by "natural remedies": an analysis of cases submitted to the Spanish Liver Toxicity Registry]. Rev Esp Enferm Dig 2008; 100: 688-95. Spanish. [PubMed: 19159172](Among 521 cases of drug induced liver injury submitted to Spanish registry, 13 [2%] were due to herbals, none of which were attributed to Garcinia cambogia).
- Chitturi S, Farrell GC. Hepatotoxic slimming aids and other herbal hepatotoxins. J Gastroenterol Hepatol 2008; 23: 366-73. [PubMed: 18318821](General review of herbal weight loss agents and hepatotoxicity includes discussions of green tea, LipoKinetix, Ma Huang and usnic acid, but not Garcinia cambogia).
- Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis 2009; 29: 373-82. [PubMed: 19826971](Review of the problems of causality assessment in herbal and dietary supplement [HDS] associated liver disease, including the variable clinical presentations, the complexity and lack of information on their components, absence of controlled trials demonstrating safety and efficacy, the possibility of contamination or incorrect labeling and frequent underreporting of herbal use by patients. Regulation of HDS is under DSHEA, which requires manufacturers to determine safety and prohibits claims of efficacy in treating specific diseases. The US Pharmacopeia sets standards for food and drugs and includes HDS; HDS induced liver injury is a growing problem and currently accounts for at least 10% of cases of acute liver injury due to medications).
- Jacobsson I, Jönsson AK, Gerdén B, Hägg S. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf 2009; 18: 1039-47. [PubMed: 19650152](Review of 778 reports of adverse reactions to herbals to a Swedish Registry; no mention of Garcinia).
- Lobb A. Hepatoxicity associated with weight-loss supplements: a case for better post-marketing surveillance. World J Gastroenterol 2009; 15: 1786-7. [PMC free article: PMC2668789] [PubMed: 19360927](Commentary on liver injury due to Hydroxycut products which contained Garcinia cambogia, summarized 6 cases in the published literature and drew attention to its hepatotoxicity and the need for better regulation and surveillance of HDS safety).
- McDonnell WM, Bhattacharya R, Halldorson JB. Fulminant hepatic failure after use of the herbal weight-loss supplement exilis. Ann Intern Med 2009; 151: 673-4. [PubMed: 19884634](25 year old man developed severe hepatitis 2 weeks after starting Exilis, a multiingredient dietary supplement similar to Hydroxycut in ingredients, including green tea and Garcinia cambogia [bilirubin 10.8 rising to 28.0 mg/dL, ALT 2362 U/L, INR rising to 3.4], ultimately requiring liver transplantation).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 [11%] were attributed to drug induced liver injury of which 12 [9%] were due to HDS, including several herbal mixtures, usnic acid, Ma Huang, black cohosh, and Hydroxycut; the possible role of Garcinia cambogia was not discussed).
- Chen GC, Ramanathan VS, Law D, Funchain P, Chen GC, French S, Shlopov B, et al. Acute liver injury induced by weight-loss herbal supplements. World J Hepatol 2010; 2: 410-5. [PMC free article: PMC3004035] [PubMed: 21173910](Report of 3 cases of acute liver injury in patients taking weight loss supplements, one due to Hydroxycut and two to Herbalife products discussing the possible role of green tea, but not Garcinia cambogia extracts in causing the injury).
- Stickel F, Kessebohm K, Weimann R, Seitz HK. Review of liver injury associated with dietary supplements. Liver Int 2011; 31: 595-605. [PubMed: 21457433](Review of liver injury from HDS focusing upon Herbalife and Hydroxycut products, green tea, usnic acid, Noni juice, and Chinese herbs, mentions that Garcinia cambogia was a primary component of Hydroxycut products before 2009).
- Onakpoya I, Hung SK, Perry R, Wider B, Ernst E. The use of Garcinia extract(hydroxycitric acid) as a weight loss supplement: a systematic review and meta-analysis of randomised clinical trials. J Obes 2011; 2011: 509038. [PMC free article: PMC3010674] [PubMed: 21197150](A systematic review of published randomized controlled trials of HCA as a weight loss agent identified 12 studies with a total of 706 subjects, found evidence of a slight effect [-0.9 kg] that was marginally significant compared to placebo while adverse events were similar between the two groups; no mention of ALT elevations or hepatotoxicity).
- Teschke R, Wolff A, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: a tabular compilation of reported cases. Liver Int 2012 32: 1543-56. [PubMed: 22928722](A systematic compilation of all publications on the hepatotoxicity of specific herbals identified 185 publications on 60 different herbs and supplements, including Garcinia cambogia which was a component of 3 proprietary multiingredient products: Herbalife, Hydroxycut and Exilis).
- Márquez F, Babio N, Bulló M, Salas-Salvadó J. Evaluation of the safety and efficacy of hydroxycitric acid or Garcinia cambogia extracts in humans. Crit Rev Food Sci Nutr 2012; 52: 585-94. [PubMed: 22530711](Review of studies in animals and humans on efficacy and safety of Garcinia cambogia as a weight loss agent; mentions that adverse effects from Garcinia extracts are uncommon and mild in humans, but that the hepatotoxicity associated with the dietary supplement Hydroxycut has been purported to be caused by the Garcinia, although the association is unproven).
- Teschke R, Schulze J, Schwarzenboeck A, Eickhoff A, Frenzel C. Herbal hepatotoxicity: suspected cases assessed for alternative causes. Eur J Gastroenterol Hepatol 2013; 25: 1093-8. [PubMed: 23510966](Review of literature of case series of suspected HDS related liver injury found evidence of other explanations in 19 of 23 publications, involving 278 of 573 patients [49%] and these other diagnoses weakened the causality assessment in most instances; no mention of Garcinia cambogia related cases).
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther 2013; 37: 3-17. [PubMed: 23121117](Systematic review of literature on HDS associated liver injury discusses Herbalife and Hydroxycut products but does not mention the possible role of Garcinia cambogia).
- Rossi S, Navarro VJ. Herbs and liver injury: a clinical perspective. Clin Gastroenterol Hepatol 2014; 12: 1069-76. [PubMed: 23924877](Review of HDS induced liver injury including regulatory problems, difficulties in diagnosis and causality assessment; mentions Herbalife products as being implicated in case series of liver injury from Israel, Switzerland and Spain and Hydroxycut products as having been implicated in many cases and the formulation changed but does not specifically mention or discuss Garcinia).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period including 15 attributed to HDS products, none of which were attributed to Garcinia cambogia).
- Licata A, Macaluso FS, Craxì A. Herbal hepatotoxicity: a hidden epidemic. Intern Emerg Med 2013; 8: 13-22. [PubMed: 22477279](Review and commentary on herbal hepatotoxicity discusses pyrrolizidine alkaloids, green tea, Echinacea, kava, usnic acid, ephedra and products made by Herbalife, Hydroxycut and LipoKinetix and lists Garcinia cambogia as a possible culprit in Hydroxycut products that caused hepatitis).
- Navarro VJ, Seeff LB. Liver injury induced by herbal complementary and alternative medicine. Clin Liver Dis 2013; 17: 715-35. [PubMed: 24099027](Review of HDS induced liver injury including regulatory problems, difficulties in diagnosis and causality assessment; mentions Herbalife and Hydroxycut products but does not mention Garcinia cambogia).
- Dağ MS, Aydınli M, Oztürk ZA, Türkbeyler IH, Koruk I, Savaş MC, Koruk M, et al. Drug- and herb-induced liver injury: a case series from a single center. Turk J Gastroenterol 2014; 25: 41-5. [PubMed: 24918129](Between 2008 and 2012, 82 patients with drug or herbal supplement induced liver injury were seen at a single referral center in Turkey, 10 [12%] of which were due to HDS products, including 7 due to Teucrium polium [mountain germander] and 3 to green tea, but none to Garcinia cambogia).
- Teschke R, Genthner A, Wolff A, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: Analysis of cases with initially reported positive re-exposure tests. Dig Liver Dis 2014; 46: 264-9. [PubMed: 24315480](Reanalysis of 34 published cases of liver injury due to herbal medications in which there was a reported positive rechallenge, finding only 21 [62%] fulfilled the criteria of a positive rechallenge using RUCAM, the others having inconsistent [18%] or incomplete data [21%]; none of the 34 cases were initially attributed to Garcinia cambogia).
- Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, Reddy KR, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology 2014; 60: 1399-408. [PMC free article: PMC4293199] [PubMed: 25043597](Among 85 cases of HDS associated liver injury [not due to anabolic steroids] enrolled in a US prospective study between 2004 and 2013, Garcinia was not mentioned among the implicated agents, although some were MIDS that list Garcinia as a component [Xenadrine, Hydroxycut).
- Navarro VJ, Lucena MI. Hepatotoxicity induced by herbal and dietary supplements. Semin Liver Dis 2014; 34: 172-93. [PubMed: 24879982](Review of HDS induced liver injury including regulatory problems, difficulties in diagnosis and causality assessment, discusses Herbalife and Hydroxycut products, but does not specifically mention Garcinia cambogia as a component of these proprietary products that might be responsible for their hepatotoxicity).
- Korth C. Drug-induced hepatotoxicity of select herbal therapies. J Pharm Pract 2014; 27: 567-72. [PubMed: 25546878](Review of liver injury due to selected HDS discusses the literature implicating kava, green tea, germander, pyrrolizidine alkaloids and Herbalife products, but does not specifically mention Garcinia cambogia).
- Semwal RB, Semwal DK, Vermaak I, Viljoen A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015; 102: 134-48. [PubMed: 25732350](Comprehensive review of Garcinia cambogia including its botanical features, geographic distribution, ethnobotanical use, phytochemistry, biologic activity, clinical uses and toxicity, including testicular injury with high doses in rats, serotonin toxicity reported in humans and rare cases of hepatotoxicity in humans which was reported largely with use of Hydroxycut products).
- Seeff LB, Bonkovsky HL, Navarro VJ, Wang G. Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology 2015; 148: 517-32. [PubMed: 25500423](Extensive review of possible beneficial as well as harmful effects of herbal products on the liver; mentions that multiingredient supplements have been implicated in many cases of liver injury including proprietary agents marketed under the names Herbalife, Hydroxycut and OxyELITE Pro, but does not specifically discuss or mention Garcinia cambogia).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a prospective database between 2004 and 2012, HDS were implicated in 145 [16%], the single major herbal cause being green tea and no cases clearly implicating Garcinia cambogia).
- Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol 2015; 89: 851-65. [PubMed: 25680499](Extensive review of liver injury due to HDS; mentions that Herbalife has been implicated in 54 cases of liver injury, 7 with a positive rechallenge, but that the cause of the injury remains unknown and that few cases have been published since 2011).
- Melendez-Rosado J, Snipelisky D, Matcha G, Stancampiano F. Acute hepatitis induced by pure Garcinia cambogia. J Clin Gastroenterol 2015; 49: 449-50. [PubMed: 25811114](42 year old woman developed severe abdominal pain one week after starting Garcinia cambogia for weight loss and while taking hydralazine for hypertension and acetaminophen/hydrocodone for back pain [bilirubin 0.3 mg/dL, ALT 1277 U/L, Alk P 283 U/L, INR 1.3], with rapid recovery on stopping all medications).
- Corey R, Werner KT, Singer A, Moss A, Smith M, Noelting J, Rakela J. Acute liver failure associated with Garcinia cambogia use. Ann Hepatol 2016; 15: 123-6. [PubMed: 26626648](52 year old woman developed anorexia and fatigue followed by jaundice 3-4 weeks after starting Garcinia cambogia [1000 mg daily] [bilirubin 8.5 mg/dL, ALT 645 U/L, Alk P 140 U/L, INR 3.2], progressing to hepatic failure and emergency liver transplantation).
- García-Cortés M, Robles-Díaz M, Ortega-Alonso A, Medina-Caliz I, Andrade RJ. Hepatotoxicity by dietary supplements: a tabular listing and clinical characteristics. Int J Mol Sci 2016; 17: 537. [PMC free article: PMC4848993] [PubMed: 27070596](Listing of published cases of liver injury from HDS products including Hydroxycut that contains Garcinia cambogia and several cases in which Garcinia was taken alone [Corey 2016 and Melendez-Rosado 2015]).
- Avigan MI, Mozersky RP, Seeff LB. Scientific and regulatory perspectives in herbal and dietary supplement associated hepatotoxicity in the United States. Int J Mol Sci 2016; 17: 331. [PMC free article: PMC4813193] [PubMed: 26950122](Overview of the US regulations regarding herbal and dietary supplements and role of FDA, Department of Agriculture, Federal Trade Commission and Office of Dietary Supplements of the NIH in assessment of safety of HDS products including actions taken against Hydroxycut which contained Garcinia cambogia extract and resulted in the change in its formulation, but cases of liver injury continued to be reported [Araujo 2015]).
- Marcus DM. Dietary supplements: What's in a name? What's in the bottle? Drug Test Anal 2016; 8 (3-4): 410-2. [PubMed: 27072845](Commentary on regulation of HDS products concludes: "the marketing of botanical supplements is based on unfounded claims that they are safe and effective", and "there is no reason to take herbal medicines whose composition and benefits are unknown and whose risks are evident").
- Lunsford KE, Bodzin AS, Reino DC, Wang HL, Busuttil RW. Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J Gastroenterol 2016; 22: 10071-6. [PMC free article: PMC5143754] [PubMed: 28018115](34 year old man developed nausea and jaundice 5 months after starting a Garcinia cambogia extract for weight loss [bilirubin 34.7 mg/dL, ALT 520 U/L, Alk P 156 U/L, INR 3.5], with progression to liver failure and successful emergency liver transplantation).
- Brown AC. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem Toxicol 2017; 107 (Pt A): 449-71. [PubMed: 27818322](Summary of the US regulations on safety and efficacy of herbal and dietary supplements, lists Garcinia cambogia as accepted into the US Pharmacopoeia monograph development process as an agent for which “available evidence does not indicate a serious risk to health”).
- Brown AC. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem Toxicol 2017; 107 (Pt A): 472-501. [PubMed: 27402097](Description of an online compendium of cases of liver toxicity attributed to HDS products, lists Exilis and Hydroxycut as containing Garcinia cambogia, among other ingredients).
- Wong LL, Lacar L, Roytman M, Orloff SL. Urgent liver transplantation for dietary supplements: an under-recognized problem. Transplant Proc 2017; 49: 322-5. [PubMed: 28219592](Among 2048 adult liver transplants recipients enrolled in the Scientific Registry of Transplant Recipients [SRTR] between 2003 and 2015, 625 were done for acute hepatic necrosis due to drug induced liver injury, half being due to acetaminophen and the 4th most frequent cause [n=21] being HDS products, while Garcinia cambogia is not specifically listed, 1 case was attributed to Hydroxycut and many listed merely as “herbal medicine” or “unknown herbals”).
- de Boer YS, Sherker AH. Herbal and dietary supplement-induced liver injury. Clin Liver Dis 2017; 21: 135-49. [PMC free article: PMC5117680] [PubMed: 27842768](Review of the frequency, clinical features, patterns of injury and outcomes of HDS hepatotoxicity with specific mention of anabolic steroids, black cohosh, germander, green tea, kava, pyrrolizidine alkaloids and proprietary multiingredient dietary supplements [MIDS]; mentions that Garcinia cambogia is an ingredient of some Herbalife products).
- Vega M, Verma M, Beswick D, Bey S, Hossack J, Merriman N, Shah A, et al.; Drug Induced Liver Injury Network (DILIN). The incidence of drug- and herbal and dietary supplement-induced liver injury: preliminary findings from gastroenterologist-based surveillance in the population of the State of Delaware. Drug Saf 2017; 40: 783-7. [PMC free article: PMC5699929] [PubMed: 28555362](A prospective, population based registry of cases of drug induced liver injury occurring in Delaware during 2014, identified 20 cases [2.7 per 100,000] overall, including 6 due to HDS products, and one of which was attributed to Garcinia cambogia).
- Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology 2017; 65: 363-73. [PMC free article: PMC5502701] [PubMed: 27677775](Review of the problems of liver injury and HDS products, specifically discusses anabolic steroids, green tea extract and OxyELITE Pro, but does not discuss Garcinia cambogia).
- Sharma A, Akagi E, Njie A, Goyal S, Arsene C, Krishnamoorthy G, Ehrinpreis M. Acute hepatitis due to Garcinia cambogia extract, an herbal weight loss supplement. Case Rep Gastrointest Med 2018; 2018: 9606171. [PMC free article: PMC6083529] [PubMed: 30147968](57 year old woman developed abdominal pain 1 month after starting Garcinia cambogia for weight loss [bilirubin 2.4 mg/dL, ALT 738 U/L, Alk P 80 U/L, INR 1.2], resolving within a month of stopping and ALT rising again [301 U/L] after restarting).
- Kothadia JP, Kaminski M, Samant H, Olivera-Martinez M. Hepatotoxicity associated with use of the weight loss supplement Garcinia cambogia: a case report and review of the literature. Case Reports Hepatol 2018; 2018: 6483605. [PMC free article: PMC5867608] [PubMed: 29721342](36 year old woman developed fatigue and jaundice 4 weeks after starting Garcinia cambogia for weight loss [bilirubin 7.4 mg/dL, ALT 5615, Alk P 104 U/L]).
- Gavrić A, Ribnikar M, Šmid L, Luzar B, Štabuc B. Fat burner-induced acute liver injury: Case series of four patients. Nutrition 2018; 47: 110-4. [PubMed: 29310849](4 case reports of acute liver injury attributed to “fat burners” used for weight loss, including spirulina [Case #1], green tea [#2], green coffee bean [#3] and a combination of green tea and Garcinia cambogia).
- Crescioli G, Lombardi N, Bettiol A, Marconi E, Risaliti F, Bertoni M, Menniti Ippolito F, et al. Acute liver injury following Garcinia cambogia weight-loss supplementation: case series and literature review. Intern Emerg Med 2018 May 25. [Epub ahead of print] [PubMed: 29802521](Four cases of acute liver injury associated with weight loss supplements, one due to spirulina, one to green tea, one to green coffee bean and one to a product with both green tea and Garcinia cambogia extract).
- Licata A, Minissale MG. Weight-loss supplementation and acute liver failure: the case of Garcinia Cambogia. Intern Emerg Med 2018; 13: 833-5. [PubMed: 30032342](Editorial in response to Crescioli [2018] summarizing the literature on Garcinia cambogia associated liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review A comprehensive scientific overview of Garcinia cambogia.[Fitoterapia. 2015]Review A comprehensive scientific overview of Garcinia cambogia.Semwal RB, Semwal DK, Vermaak I, Viljoen A. Fitoterapia. 2015 Apr; 102:134-48. Epub 2015 Feb 27.
- Evaluation and Characterization of Malabar Tamarind [Garcinia cambogia (Gaertn.) Desr.] Seed Oil.[J Food Sci Technol. 2015]Evaluation and Characterization of Malabar Tamarind [Garcinia cambogia (Gaertn.) Desr.] Seed Oil.Choppa T, Selvaraj CI, Zachariah A. J Food Sci Technol. 2015 Sep; 52(9):5906-13. Epub 2014 Dec 12.
- Screening of microalgae for treating Garcinia cambogia wash water with potential lipid production.[Environ Sci Pollut Res Int. 2019]Screening of microalgae for treating Garcinia cambogia wash water with potential lipid production.Budhi Venkatesan R, Rajarathinam R. Environ Sci Pollut Res Int. 2019 Dec; 26(34):34685-34692. Epub 2018 Jun 29.
- Review Acute liver injury following Garcinia cambogia weight-loss supplementation: case series and literature review.[Intern Emerg Med. 2018]Review Acute liver injury following Garcinia cambogia weight-loss supplementation: case series and literature review.Crescioli G, Lombardi N, Bettiol A, Marconi E, Risaliti F, Bertoni M, Menniti Ippolito F, Maggini V, Gallo E, Firenzuoli F, et al. Intern Emerg Med. 2018 Sep; 13(6):857-872. Epub 2018 May 25.
- Garcinia cambogia, Either Alone or in Combination With Green Tea, Causes Moderate to Severe Liver Injury.[Clin Gastroenterol Hepatol. 2022]Garcinia cambogia, Either Alone or in Combination With Green Tea, Causes Moderate to Severe Liver Injury.Vuppalanchi R, Bonkovsky HL, Ahmad J, Barnhart H, Durazo F, Fontana RJ, Gu J, Khan I, Kleiner DE, Koh C, et al. Clin Gastroenterol Hepatol. 2022 Jun; 20(6):e1416-e1425. Epub 2021 Aug 14.
- Garcinia Cambogia - LiverToxGarcinia Cambogia - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...