Abbreviations
- FHR
fetal heart rate
- IOL
induction of labour
- kg
kg
- mcg
micrograms
- PROM
premature rupture of membranes
- RCT
randomized controlled trial
- SOGC
Society of Obstetricians and Gynaecologists of Canada
- UK
United Kingdom
- US
United States
Context and Policy Issues
Induction of labour (IOL) is the process of initiating contractions in pregnant persons who are currently not in labour, to help them achieve vaginal delivery within 24 to 48 hours.1 Cervical ripening is one of the methods used for labour induction; it is “the use of pharmacological or other means to soften, efface, or dilate the cervix to increase the likelihood of a vaginal delivery.”1 (p. 843) The two major techniques for cervical ripening are mechanical interventions (e.g. insertion of balloon catheters), and application of pharmacological agents (e.g. prostaglandins).2 Prostaglandins are one of the preferred methods for cervical ripening, including the agents dinoprostone and misoprostol.3
Labour induction now exceeds 20% of all births in most countries.3 In Canada, the rate of labour induction increased from 12.5% in 1991/1992 to a high of 23.7% in 2001/2002. The rate decreased to 21.8% in 2004/2005.1
Induction of labour is indicated when the risk of continuing the pregnancy outweighs the risks of labour induction and delivery for the birthing parent or the fetus. Examples of more urgent reasons for induction include preeclampsia at 37 weeks or more, chorioamnionitis, significant illness during pregnancy that is unresponsive to treatment, suspected fetal compromise, and term pre-labour rupture of membranes (PROM) with maternal group B streptococcus colonization. Induction is contraindicated in previous uterine rupture, pelvic structural deformities, and abnormal fetal lie or presentation. Labour induction increases the risk of Caesarean section and surgical vaginal delivery, chorioamnionitis, cord prolapse with artificial rupture of membranes, and uterine rupture in scarred and unscarred uteri. Even though undergoing labour induction, some may also fail to achieve labour.1
Misoprostol is a synthetic analogue of prostaglandin E1, which has gastric antisecretory and mucosal protective effects. The oral form is approved in Canada for the treatment and prevention of gastroduodenal ulcers caused by nonsteroidal anti-inflammatory drugs (NSAIDs), and for the treatment of duodenal ulcers caused by peptic ulcer disease.4 The most common side effects with a single oral dose of misoprostol are diarrhea, abdominal pain, nausea, flatulence, and dyspepsia.4
Misoprostol also has uterotonic properties, by contracting smooth muscle fibers in the myometrium and relaxation of the cervix, facilitating cervical opening.5 It is approved in Canada for the termination of intrauterine pregnancy with a gestational age of 63 days or less, in combination with mifepristone.
Misoprostol tablets, administered orally and vaginally, are used for the induction of labour or cervical ripening, but are not currently approved by Health Canada for this indication.6,7 The usual dose is 50 mcg orally or 25 mcg vaginally, which may be repeated every 4 hours if contractions are absent or not painful.1 Serious adverse effects with misoprostol for cervical ripening and labour induction are similar to other prostaglandins, and include uterine tachysystole, meconium staining of liquor, and rarely, uterine rupture.1 Other side effects include fever, chills, vomiting, and diarrhea.6
Concerns about using misoprostol for induction of labour in Canada included the lack of approval for this indication by Health Canada, and the risk of serious adverse events like uterine rupture. This review aims to review the clinical effectiveness, cost-effectiveness, and evidence-based guidelines regarding the use of misoprostol for cervical ripening and induction of labour.
Research Questions
What is the clinical effectiveness of misoprostol for cervical ripening and induction of labour?
What is the cost-effectiveness of misoprostol for cervical ripening and induction of labour?
What are the evidence-based guidelines regarding misoprostol for cervical ripening and induction of labour?
Key Findings
Evidence from meta-analyses, Canadian guidelines, and randomized controlled trials suggests that misoprostol is likely effective and safe for induction of labour and cervical ripening. Limitations include the heterogeneity between administration methods and dosages of induction regimes (both misoprostol and comparators). Economic trials indicate that titrated oral low-dose misoprostol and buccal/sublingual misoprostol are cost-effective methods for induction of labour when compared to other pharmacological interventions; however none of the cost studies were performed in the Canadian setting.
Methods
Literature Search Methods
A limited literature search was conducted on key resources including Ovid Medline, PubMed (for non-Medline records), the Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. Methodological filters were applied to limit retrieval to health technology assessments, systematic reviews, meta-analyses, randomized controlled trials, non-randomized studies, economic studies, and guidelines. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2013 and October 23, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in :
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2013. Systematic reviews and meta-analyses were excluded if the reviewed articles overlapped with another review or meta-analysis. Randomized controlled trials (RCTs) were excluded that were published prior to the earliest publishing date of the included systematic reviews (2016) were also excluded. Guidelines with unclear methodology were also excluded.
Critical Appraisal of Individual Studies
The included systematic reviews were critically appraised by one reviewer using AMSTAR 28, randomized studies were critically appraised using the Cochrane risk-of-bias tool for randomized trials,9 economic studies were assessed using the Drummond checklist,9 and guidelines were assessed with the AGREE II instrument.10 Summary scores were not calculated for the included studies; rather, a review of the strengths and limitations of each included study were described narratively.
Summary of Evidence
Quantity of Research Available
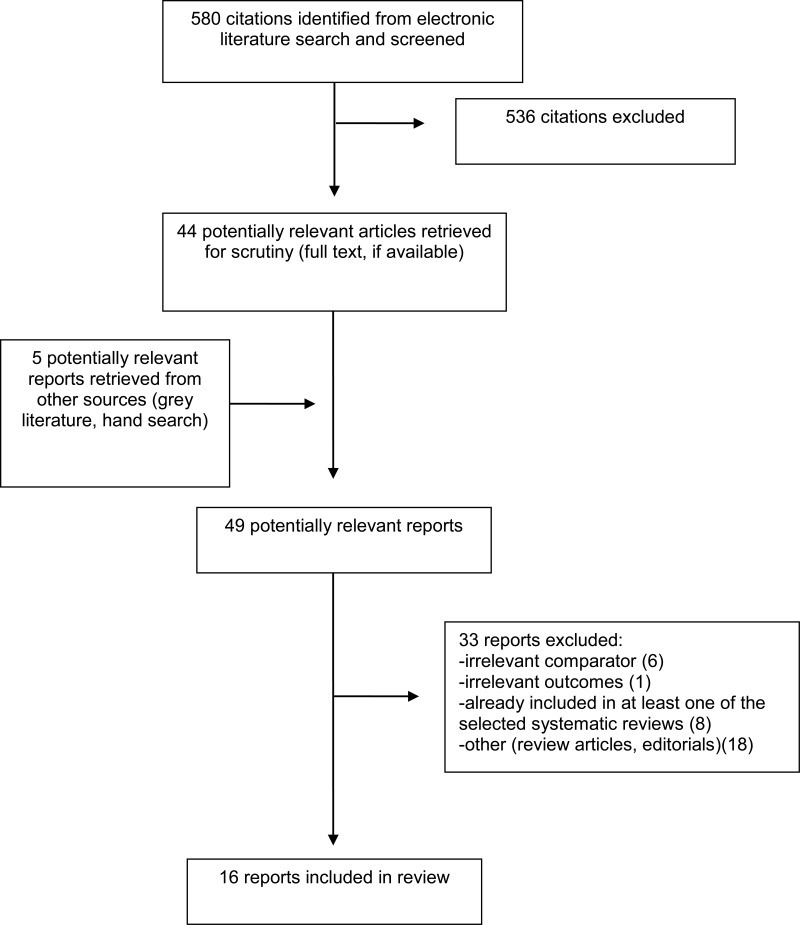
A total of 580 citations were identified in the literature search. Following screening of titles and abstracts, 536 citations were excluded and 44 potentially relevant reports from the electronic search were retrieved for full-text review. Five potentially relevant publications were retrieved from the grey literature search for full text review. Of these potentially relevant articles, 33 publications were excluded for various reasons, and 16 publications met the inclusion criteria and were included in this report. These comprised two systematic reviews, nine RCTs, four economic evaluations, and one evidence-based guideline. Appendix 1 presents the PRISMA11 flowchart of the study selection.
Summary of Study Characteristics
Study Design
Two systematic reviews with meta-analysis met the inclusion criteria for the clinical effectiveness of misoprostol in induction of labour.12,13 The Alfirevic review was published in 2016, and the authors searched for RCTs that examined pharmacological, mechanical, and complementary medicine methods for induction of labour, with no restriction on languages, published up to March, 2014. Databases searched included Medline, Embase, Cochrane Library, CINAHL, and they also searched trials registry portals, hand-searched journal and conference proceedings, and reviewed selected journals not included in other databases. The review included 611 trials; 141 (N = 28,845) trials were evaluated for vaginal delivery not achieved within 24 hours; 586 trials (N = 96,771) were evaluated for risk of cesarean section; 181 trials were assessed for uterine hyperstimulation with fetal heart rate changes (N = 43,612); and 299 trials (N = 54,511) were assessed for the risk of instrumental delivery.12
The ten Eikelder review was published in 2017, and the authors searched in Pubmed, Embase, Cochrane Library, and Web of Science for RCTs. The review included 22 trials (N = 5, 015).13 There was overlap between the Alfirevic and ten Eikelder reviews; Appendix 5, presents the overlap between these reviews.
There were nine RCTS that met the inclusion criteria. There was one single-blind RCT,14 seven open-label randomized trials,15–21, and one double-blind RCT.22
There were four economic evaluations that met the inclusion criteria.
Leigh et al.23 used clinical data from the Mundle16 trial and cost data from an Indian medical college, for an Indian healthcare system perspective. The authors performed a cost-consequences analysis, and the main assumptions were a vaginal delivery rate of 41% with Foley catheter, that midwives administered misoprostol at 2-hourly intervals, and that financial costs for staff time were equivalent.23
Bierut et al.24 performed a meta-analysis on 11 trials pertaining to the induction of labour (IOL) for clinical data. The investigators used a cost-consequences model for economic assessment, and the main assumptions were that the patient spends time before active labour in the antenatal ward and then enters the labour ward during labour, that IOL can end with vaginal delivery or cesarean section, and that patient and neonates are in the postnatal ward until discharge. The cost data was based on retrospective data collection (from a questionnaire) for unit costs and resource utilization from 5 countries.24
Draycott et al.25 used published and non-published data from the EXPEDITE study for the clinical measures, and cost data from the Southmead Hospital in Bristol, UK. It was a short-term study with a UK National Health Services perspective, and investigators used a Markov model. The main assumptions were a cycle length of one hour, different labour induction methods were mutually exclusive, all participants received only one of the comparators, the onset of labour and delivery were a function of the time since induction, and the non-Southmead Hospital demographics were assumed to be identical to the EXPEDITE population.25
The Alfirevic12 cost-effectiveness study used a decision-analytic model for a UK perspective, for short-term costs. The source of clinical data was from a network meta-analysis, and the costs were the average expenditures from the UK National Health Service for intervention, labour delivery method, and length of neonatal stay. The main assumptions were that relative effects for NICU admission were independent of delivery timing but dependent on delivery mode, that the probability of NICU admission was1.5 times higher for cesarean section, that vaginal delivery within 24 hours was defined as short stay and more than 24 hours as long stay (including cesarean section), and most cost differences between IOL methods occur during and immediately after birth.12
ten Eikelder et al.26 performed a cost-effectiveness analysis, with a short-term time horizon and Dutch hospital perspective. The authors used clinical data from 1,845 participants in the PROBAAT-2 trial,27 and collected resource data from the case record forms, medication costs from the Dutch Pharmacotherapeutic Compass, and cost of induction methods from a university medical centre hospital pharmacist. The main assumptions were that the duration of labour ward occupation was the interval between admission to the labour ward and birth plus 1 hour for recovery care, and the costs for a cesarean section were the costs of 1 hour in the operating room.26
There was one guideline that met the inclusion criteria regarding the use of misoprostol for the induction of labour, the 2013 document from the Society for Society of Obstetricians and Gynaecologists of Canada (SOGC).1 The guideline included systematic reviews, RCTs, controlled clinical trials, and observations studies; there were no date or language restrictions. The guideline used the evidence statements and grading of recommendations adapted from the Canadian Task Force on Preventive Health Care to assess the evidence. The guideline did not indicate which method was used to make recommendations (e.g. consensus, voting).1
Country of Origin
The lead author for the Alfirevic systematic review is from the UK, and the lead author for the ten Eikelder review is from the Netherlands.12,13
Lead authors for the RCTs were from China21, Pakistan15, India16, Kenya17, United States14,18, Iran 19, and Saudi Arabia.20
The lead author for the misoprostol vaginal insert economic model is from Poland24, Netherlands for the misoprostol cost-effectiveness comparison with Foley cathers26, and the lead authors for the Draycott, Alfirevic, and Leigh studies were from the UK.12,23,25
The SOGC guideline’s recommendations are for Canadian obstetrical care providers.1
Patient Populationa
The eligibility criteria for the Alfirevic review were studies that included pregnant women carrying a viable fetus, and who were eligible for any method of third-trimester cervical ripening or labour induction.12 The ten Eikelder review reviewed studies that included pregnant women scheduled for third trimester induction of labour, who had an unfavorable cervix, a viable fetus in cephalic presentation, and who did not have a prior cesarean delivery.13
The patient population in the RCTs included:
Pregnant women at a hospital in Montevideo, Uruguay, with gestational ages between 32/0 and 41/6, a viable fetus in cephalic presentation, an estimated fetal weight less than 4 kg, and no contraindication for vaginal birth.
14Pregnant women at a hospital in Karachi, Pakistan, aged 20 to 40 years, with a singleton pregnancy and a fetus in cephalic presentation.
15Pregnant women at two public hospitals in Nagpur, India, who were at least 18 years of age, at 20 weeks gestation or later with a live fetus, who required IOL due to pre-eclampsia or hypertension.
16Pregnant women at a hospital in Nairobi, Kenya, with gestational age 28 weeks or more with indications for IOL (late-term or post-term pregnancies, pre-eclampsia, chronic hypertension, gestational diabetes, oligohydramnios, or intrauterine fetal demise), Bishop score less than 6, singleton cephalic pregnancy, intact membranes, and no contraindications to vaginal delivery.
17Nulliparous and multiparous English-speaking women at a hospital in Bronx, New York, aged 18 to 50 years, with singleton term pregnancies of 37 weeks or more gestation, and with a Bishop score less than 6.
18Pregnant women at two academic hospitals in Iran, with premature rupture of membranes (PROM) between 37 and 42 weeks of gestation, singleton pregnancy, cephalic presentation, history of fewer than five vaginal deliveries, no hypersensitivity to prostaglandins, no contraindication to vaginal delivery, no other cervical ripening methods within 7 days of hospitalization, no previous uterine scar, Bishop scores of 6 or less (unripe cervix), and reassuring fetal heart rate patterns.
19Pregnant women at a hospital in Saudi Arabia indicated for IOL with a singleton live pregnancy, 34 weeks gestation or more, Bishop score ≤ 6, intact membranes, cephalic presentation, and a reassuring fetal heart rate.
20Pregnant women at 4 obstetrical centres in China, with singleton pregnancies in occipital presentation, nullipara, gestational age 36 weeks or more, Bishop score less than 6, and no contraindication to vaginal delivery.
21Pregnant women at a teaching hospital in India undergoing IOL between 37 and 42 weeks gestation, singleton pregnancy in cephalic presentation, Bishop score less than 6, intact membranes, and a reassuring fetal cardiotocograph.
22
For the economic analyses, the Bierut study24 literature search identified studies with women undergoing induction of labour. No other details were reported in the study.24 The patients in the Draycott study25 were women undergoing induction of labour. The Alfirevic study12 included pregnant women carrying a viable fetus who were eligible for any method of third trimester labour induction. The ten Eikelder cost-effectiveness comparison26 included women with a viable singleton pregnancy in cephalic presentation, intact membranes, a gestational age of 37 weeks or more, no previous caesarean section, a Bishop score less than 6, and who were scheduled for induction of labour. The Leigh economic evaluation23 used trial data for pregnant patients requiring induction of labour and delivery for hypertension or pre-eclampsia.
The SOGC guideline is relevant to the care of pregnant women undergoing induction of labour; no other information was provided. The intended users are obstetrical care providers.1
Interventions and Comparators
The Alfirevic systematic review12 included induction of labour studies with no treatment, placebo, all pharmacological methods at all routes and doses (misoprostol, dinoprostone, dinoprost, oxytocin, nitric oxide, mifepristone, estrogens, corticosteroids, relaxin, and hyaluronidase), mechanical methods (catheters, laminaria tents, membrane sweep, and amniotomy), and complementary medicine methods. The misoprostol doses reviewed included oral and vaginal doses less than 50 mcg as well as 50 mcg and more.12
The ten Eikelder review included studies that compared Foley catheter with misoprostol at any dose or route of administration, and studies comparing Foley catheter plus misoprostol versus misoprostol alone.13
The interventions and comparators in the RCTs were:
For the economic analyses:
The interventions in the Leigh study were transcervical Foley catheters compared with oral misoprostol 25 mcg administered every 2 hours, for a total of 12 doses or until active labour started.
23The interventions for the Bierut study were misoprostol vaginal inserts compared with dinoprostone vaginal inserts, dinoprostone cervical gel, oxytocin, and Foley or Cook catheters.
24The interventions for the Draycott study were misoprostol vaginal inserts compared with dinoprostone vaginal inserts.
25The interventions included in the Alfirevic cost-effectiveness study were: placebo; vaginal and oral misoprostol less than 50 mcg and 50 mcg and more; titrated low-dose oral misoprostol; sustained-release (SR) misoprostol vaginal inset; buccal/sublingual misoprostol; vaginal dinoprostone tablets, gel, and SR pessaries; extra-amniotic dinoprostone; IV oxytocin with or without amniotomy; nitric oxide; mifepristone; Foley catheter; and double-balloon or Cook’s catheter.
12The interventions in the ten Eikelder cost-effectiveness study were oral misoprostol 50 mcg every 4 hours (total of 3 doses per 24 hours) compared with Foley catheter.
26
The pharmacological interventions considered in the 2013 SOGC guidelines for cervical ripening and induction of labour in patients with an unfavorable cervix were cervical and vaginal dinoprostone (prostaglandin E2), and misoprostol.1
Outcomes
Labour delivery outcomes
The primary outcome for the Alfirevic meta-analysis12 was vaginal delivery not achieved within 24 hours.12
The primary outcomes for effectiveness of IOL in the ten Eikelder systematic review were total cesarean delivery rate, cesarean delivery for failure to progress first stage, and total vaginal instrumental deliveries.13
Secondary outcomes in the Conde study included frequency of vaginal birth and cesarean section, frequency for new misoprostol doses needed to continue induction of labour, and the frequency of labour diagnosis 6 hours after misoprostol administration.14
The primary outcome in the Husain trial15 was rate of failure to achieve vaginal delivery within 24 hours of labour induction with either intervention. The secondary outcomes included induction-to-delivery interval, mode of delivery (vaginal or cesarean), reason for cesarean section, maternal complications, and NICU admissions.15
The primary outcome in the Mundle trial was vaginal birth within 24 hours. Secondary outcomes were induction-to birth interval, vaginal births within 12 hours, cervix unchanged at 12 hours and 24 hours, oxytocin augmentation, time from randomisation to start of induction and birth, total doses of misoprostol, and number of participants given a 50 mcg dose.16
Secondary outcomes in the Otosi study included frequency of vaginal birth and cesarean section, frequency of new misoprostol doses needed to continue IOL, and frequency of labour diagnosis 6 hours after misoprostol administration.17
The primary outcome in the Pimentel trial was rate of failure to achieve vaginal delivery 24 hours of IOL with either a single or multiple dose of vaginal misoprostol. Secondary outcomes included induction-to-delivery interval, mode of delivery, and reason for cesarean section.18
The primary outcome in the Pourali trial was mean time between the start of labour induction and the start of active labour. Secondary outcomes included duration of active labour, and duration of second stage of labour.19
The primary outcome in the Rouzi study was vaginal delivery within 24 hours after the treatment was started. Secondary efficacy outcomes were rate of cesarean delivery and oxytocin requirements.20
The outcomes in the Wang trial included labour induction, mode of delivery, induction-to-delivery time, total misoprostol dose, and oxytocin use.21
The primary outcome in the Yenuberi study was vaginal delivery within 24 hours of induction. Secondary outcomes included duration from IOL start to delivery, vaginal delivery within 12 hours, delivery by lower segment cesarean section, oxytocin augmentation, and the total number of misoprostol doses.22
Bishop score
The primary outcome in both the Conde and Otosi studies was Bishop score variation of at least 2 points at 6 hours after misoprostol administration.14,17 Secondary outcomes of the Yenuberi study included Bishop score at amniotomy.22
Safety outcomes during induction and delivery
The primary measures of safety for the Alfirevic meta-analysis12 were uterine hyperstimulation with FHR changes and cesarean section. These results were reported as a network meta-analysis (NMR), with odd ratios and a 95% credible interval. Secondary outcomes included serious maternal morbidity or death.28
Primary safety outcomes for the ten Eikelder review included uterine hyperstimulation, cesarean delivery for non-reassuring FHR, and vaginal instrumental delivery for non-reassuring FHR, postpartum hemorrhage.13
Maternal complications or morbidity were part of the secondary outcomes in the Husain, Mundle, Pimentel, Wang, and Rouzi trials.15,16,18,20,21
Tachysystole was a secondary outcome in the Conde and Osoti studies.14,17
Secondary outcomes in the Pourali trial included misoprostol and oxytocin side effects.19
Neonatal or fetal safety outcomes
Secondary outcomes for the Alfirevic meta-analysis included serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.12
The primary fetal and neonatal safety outcomes for the ten Eikelder meta-analysis13 were meconium stained liquor, arterial umbilical cord pH of less than 7.05, Apgar score of less than 7, NICU admission, and neonatal mortality.
Fetal and neonatal complications were included in secondary outcomes for the Mundle and Rouzi trials.16,20 NICU admission was a secondary outcome in the Pimentel and Husain studies.15,18
Outcomes in the Wang trial included fetal tachycardia, meconium-stained liquor, and Apgar scores less than 7 at 1, 5, and 10 minutes.21
Other outcomes
Participants’ expectations about labour were included in secondary outcomes for the Mundle trial.16 The Alfirevic meta-analysis included maternal satisfaction with induction methods in its outcomes.12
A detailed summary is provided in Appendix 2
and for SRs and RCTs respectively.
Economic outcomes
The Bierut economic study.24 time to delivery and time to active labour endpoints were based on a meta-analysis of 11 trials. The main outcomes of the model were total real-world cost differences of MVI versus the comparator/patient, and the proportion of patients with cost and utilization results for vaginal and cesarean delivery.24
The Draycott economic outcomes were resource utilization during pre-active labour, active labour, delivery, and inpatient stay.25 The Alfirevic cost-effectiveness primary outcomes were maternal and neonatal costs for rate of vaginal delivery within 24 hours, cesarean section rate, frequency of admission to the NICU, and resource use and utilities.12
The outcomes in the ten Eikelder cost-effectiveness study were composite safety outcomes (asphyxia and postpartum hemorrhage), and cesarean section for effectiveness measures.26
The primary clinical outcome in the Leigh economic evaluation23 was vaginal delivery within 24 hours of induction. The cost-consequences analysis compared vaginal delivery between the interventions, delivery by all methods within 24 hours of induction, and vaginal delivery within 24 hours of induction.23
A detailed summary of the economic studies is provided in Appendix 2, .
The 2013 SOGC guidelines make recommendations for misoprostol use in induction of labour, contraindications to its use in this setting, and when oxytocin augmentation should be started. The guidelines also provide information on dosing, side effects, and fetal monitoring.1
A detailed summary for the guideline is provided in Appendix 2, .
Summary of Critical Appraisal
Systematic reviews
The systematic reviews were assessed using the AMSTAR 2 tool.8 Both the Alfirevic and ten Eikelder review include a clearly defined research question (relevant population, intervention, comparators, and outcomes). For both Alfirevic and ten Eikelder reviews, the study selection and data extractions were done in duplicate. The Alfirevic review clearly outlined the rationale for excluding studies from the analysis, listing the reasons for exclusion, and they also described the studies included in the review in sufficient detail. The ten Eikelder review did not include a list of excluded studies nor provide details on included studies; unjustified exclusion could bias the review results, and users also unable to make judgments on included studies for relevance to their practice or appropriateness. Both studies assessed the risk of bias, as per the Cochrane Handbook for Systematic Reviews (v. 5.1.0), and the authors excluded studies of unclear or high risk of bias. The Alfirevic review performed a network meta-analysis (indicated for indirect comparisons as seen in this paper) within a Bayesian framework using OpenBUGS (v. 3.2.3) ten Eikelder used RevMan (v. 5.3), and both review discussed heterogeneity in the results.12,26
Both the ten Eikelder and Alfirevic reviews excluded non-RCTs, which could cause potential bias in the results if different outcomes from non-RCTs were not included. The ten Eikelder review performed subgroup analyses for different misoprostol doses, which was not part of the primary outcomes. The investigators for both reviews did not search the reference lists and bibliographies of the included studies, which may not have identified studies that met criteria. The Alfirevic authors did not report on publication bias, nor did they report their own competing interests, and the ten Eikelder authors did not include funding sources for the included studies; all of these could conceal bias.12,13
The Alfirevic meta-analysis12 was also hindered by data missing from included trials, including the number of participants who did not give birth vaginally within 24 hours, and key safety outcomes like uterine hyperstimulation, low Apgar score, and serious infant morbidity or mortality. There was also uncertainty around the effects estimates due to the high heterogeneity amongst results.12
Another limitation of the ten Eikelder systematic review13 includes a wide variability in the population characteristics (e.g. Bishop score, pregnancy duration), the interventions were not equal (e.g. differences in Foley catheter balloon size and volume, various misoprostol routes and doses), and there were different regimes of total induction time, which could influence mode of delivery.13
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
Clinical studies
The nine RCTs were assessed using the Cochrane risk-of-bias tool (RoB 2.1)9
Although the open-label studies were randomized, there was a risk of bias from lack of blinding to the interventions. Due to the nature of the various dosage forms, most caregivers/outcome assessors administering the drug were not blinded to the intervention15,16,18–21. Only in the Osoti trial, outcome assessors were not blinded but the study statistician was blinded.17 The Conde trial did not report if assessors were blinded, although the interventions were administered by a technician.14 One trial was a randomized, double-blind trial, with low risk of bias from blinding.22
In most of the trials, patients received the assigned intervention14–17,19–22 However, in one study some patients were switched to the alternative treatment due to caregiver preference, which leads to bias in the results.18 For patients who were randomized but did not receive treatment, intention-to-treat (ITT) analysis was performed in four trials14,16,20,22, and four studies did not do an ITT analysis.15,18,19,21 Co-interventions (e.g. oxytocin) were similar across the groups in six studies,14,16,18,20–22, but this information was not reported in two trials.17,19 Authors of one study did not state if there were co-interventions.29
Most studies reported all outcomes, or analyzed the results as per intention-to-treat.14,16–18,20,22 Three trials were at risk of bias affecting the results, as outcomes were not reported for randomized patients who were later excluded from the study.15,19,21 One paper did not include all randomized patients in the secondary outcomes.19
The measurements of outcomes were appropriate for most studies.14,15,17,18,20–22 However, lack of blinding of assessors in the open-label studies could lead to bias in favor of interventions over comparators, or vice versa.14–21
Most studies reported on the primary and secondary outcomes from the trial design, although most of the trials were not powered to detect differences in the secondary outcomes.14,15,18–20,22. However, four studies reported on additional outcomes or stratified results that were not part of the original primary and secondary outcomes. These types of measurements may favor the intervention over the comparator.15–17,19
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
Economic studies
All studies stated their research question and the alternative(s) were described.12,23–26 Four studies stated the economic importance of the research question,12,23,25,26 but this was not included in the Bierut study.24 Four studies justified the use of their economic mode and stated or included the time horizon and benefits12,23,25,26 but the Bierut authors did not discuss the reasons for the choice of economic model, nor the time horizon of the costs and benefits.24 All studies clearly stated the primary economic outcomes and methods to value benefits.12,23–26
Two studies performed sensitivity and incremental analyses.12,26 The Draycott and Leigh authors performed a sensitivity analysis but not an incremental analysis, and the Bierut authors did neither analysis.23–25 For three studies the main question was answered and the conclusions followed from the data reported12,23,26 but neither the Draycott nor Bierut studies included the cost of the main comparator in their conclusions.24,25 The Bierut study also did not discuss all of the data that was reported, leading to potential bias.24 Both the Bierut and Draycott studies were funded by the manufacturer of the main comparator (misoprostol vaginal insert), and both articles had authors who were employed by the manufacturer; this is a potential source of bias.24,25
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
Guideline
The 2013 SOGC guideline1 was assessed using the AGREE II tool.10 The guideline described the overall objective, and the population, target users, and health questions were described. The guidelines did include a systematic search for studies and grey literature, but there was no description of inclusion/exclusion criteria for the studies, which could be a source of bias. The strengths and limitations of the evidence were described, but not the methods for formulating the recommendations. The authors did not state if the guideline development group had people from all relevant professional groups, nor whether they sought opinions from patients or the public. The recommendations are specific and unambiguous and clearly outlined. They include the benefits, side effects, and risks, and are supported by the evidence. The recommendations also include different options for the induction of labour. However, there is no information on whether the guideline was externally reviewed by experts, if there is an update procedure, facilitators and barriers to its application, how recommendations can be implemented, potential resource implications, or any monitoring criteria. Implementation information and resource implications would be beneficial for Canadian sites planning on using misoprostol in their practice, as would a guideline that incorporates newer evidence. The authors also did not declare any competing interests, and it is unknown if the funding body influenced the recommendations, which could be a source of bias.1
Additional details regarding the strengths and limitations of this guideline are provided in Appendix 3.
Summary of Findings
Clinical Effectiveness of Misoprostol for Cervical Ripening and Induction of Labour
Vaginal delivery within 24 hours
For induction of labour, the Alfirevic network meta-analysis12 suggested that oxytocin with amniotomy and misoprostol vaginal doses of 50 mcg or more were more effective than other agents in achieving vaginal delivery within 24 hours. However, the oxytocin with amniotomy trials predominantly recruited women with a favorable cervix. The misoprostol low-dose oral solution and buccal/sublingual tablets performed better than both the high-dose and low tablets. For failure to achieve vaginal delivery within 24 hours, the odds ratio (OR) favored the pharmacological and mechanical interventions compared with placebo. The OR for vaginal misoprostol doses of 50 mcg or more (OR 0.09) was less than other comparators when compared to placebo, including low-dose oral misoprostol (OR 0.1), vaginal misoprostol doses less than 50 mcg (OR 0.11), vaginal dinoprostone gel (OR 0.13), oral misoprostol doses of 50 mcg or more (OR 0.16), intracervical dinoprostone (OR 0.18), Foley catheter (OR 0.19), and oral misoprostol less than 50 mcg (OR 0.22); these results were statistically significant. However, the authors stated that the wide 95% credible intervals for these rankings indicated considerable uncertainty. The authors concluded that all interventions, even with high heterogeneity between trials, increased the probability of vaginal birth within 24 hours, except for extra-amniotic dinoprostone. The authors were not able to make any conclusions regarding maternal satisfaction with IOL methods due to lack of data.12
Cesarean section and instrumental delivery
The Alfirevic meta-analysis12 concluded that the odds of patients having a cesarean section were reduced with the use of titrated low-dose misoprostol, vaginal misoprostol at doses less than 50 mcg to 50 mcg or more, vaginal dinoprostone gel, intracervical dinoprostone, oral misoprostol doses of 50 mcg or more, Foley catheters, membrane sweeping, and buccal/sublingual misoprostol; these results were statistically significant. Oral misoprostol doses less than 50 mcg did not reduce the risk of a cesarean section compared to placebo.12
A 2017 study (n = 102) concluded that misoprostol 50 mcg every four hours for four doses plus Foley catheter reduced the risk of cesarean section compared to misoprostol alone.15
For reduction in instrumental delivery, vaginal dinoprostone slow-release pessary and Foley catheter interventions significantly reduced this outcome compared to placebo; none of the misoprostol formulations reduced this outcome significantly compared to placebo.12
Bishop score
One RCT (n = 102) found no statistically significant difference in Bishop score between sublingual misoprostol 50 mcg and vaginal misoprostol 50 mcg at 6 hours after administration (P = 0.761), nor were there significant differences in the need for additional doses for labour induction, presence of complications, mode of delivery, Apgar score, or umbilical cord pH. For both routes of administration, there was a significant difference in Bishop score 6 hours after misoprostol administration (P < 0.05). The study size was not adequate to show further differences, as seen in the large confidence intervals.14
Misoprostol compared with dinoprostone
One RCT (n = 401) compared oral misoprostol solution with vaginal dinoprostone (n = 411). The results showed that titrated low-dose oral misoprostol solution was as effective for labour induction as dinoprostone, with similar maternal and neonatal outcomes. Misoprostol caused significantly less hyperstimulation and non-reassuring fetal heart rate changes than dinoprostone.21
Misoprostol compared to Foley catheters
Results from the ten Eikelder meta-analysis showed that Foley catheters were associated with less uterine hyperstimulation, fewer vaginal instrumental deliveries, and fewer cesarean deliveries for non-reassuring FHR than misoprostol at any dose or route. There were comparable total cesarean delivery rates for both interventions, and no differences for neonatal outcomes (arterial umbilical cord pH less than 7.05, Apgar less than 7 at 5 minutes, and NICU admission rates). Some limitations of the review were that was a wide variability in the population characteristics (e.g. Bishop score, pregnancy duration), the interventions were not equal (e.g. differences in Foley catheter balloon size and volume, various misoprostol routes and doses), and there were different regimes of total induction time, which could influence mode of delivery. The authors concluded that while the review showed a better safety profile for Foley catheters compared to misoprostol, the numbers were too low for a definite conclusion.13
One RCT (n = 102) concluded that participants receiving misoprostol 50 mcg every four hours for four doses plus Foley catheter were more likely to deliver vaginally within 24 hours compared to misoprostol alone. For maternal and neonatal complications, the only statistically significant difference was that more neonates in the misoprostol group had an Apgar score less than seven.15
Another RCT (n = 180) found no statistical difference in failed induction of labour between Foley catheter plus vaginal misoprostol 25 mcg and vaginal misoprostol 25 mcg alone. The Foley combination shortened the induction-to-delivery time compared to vaginal misoprostol alone. There was also no significant difference for maternal or perinatal outcomes between the two interventions.17
A larger RCT (n = 602) in participants with pre-eclampsia or hypertension concluded that vaginal birth within 24 hours was more common for those receiving oral misoprostol 25 mcg every 2 hours than in those in the Foley catheter group. For both groups, rates of uterine hyperstimulation, FHR abnormality, and severe hypertension were low.16
Misoprostol compared to oxytocin
One RCT (n = 240) compared sublingual misoprostol with oxytocin IV started at 2 milliunits/minute (titrated by 2 milliunits/minute every 20 minutes until adequate contractions. There was no significant difference between the interventions for the time between IOL and active labour (P = 0.299). The misoprostol group reported higher rates of uterine tachysystole and FHR abnormalities.19
Misoprostol comparative doses
One study (n = 243) compared single dose of vaginal misoprostol 25 mcg with vaginal misoprostol 25 mcg every four to six hours for a total of four doses. The authors found no difference between the groups in the number of participants who delivered vaginally within 24 hours, nor a difference in the Bishop score before starting oxytocin. There was no significant difference between maternal and neonatal adverse outcomes between the two groups.18
A study comparing hourly titrated oral misoprostol doses with a fixed every 2 hour oral misoprostol regimen (n = 146) found no difference for vaginal delivery within 24 hours, but the rates of cesarean section, uterine tachysystole, and meconium-stained fluid in neonates were higher in the hourly titrated group.20
Another study compared compared three doses of vaginal misoprostol 25 mcg every four hours for three doses, with oral misoprostol every four hours for three doses (50 mcg for first dose then 100 mcg). Vaginal delivery within 24 hours was similar between the two groups, but the oral misoprostol group was less likely to use oxytocin to augment labour. The groups reported similar results in mean duration form IOL to delivery, mode of delivery, and fetal/neonatal and maternal outcomes.22
Uterine Hyperstimulation
The Alfirevic meta-analysis12 showed that compared with placebo, IV oxytocin with amniotomy (OR 7.44), slow-release misoprostol vaginal pessary (OR 5.58) and high-dose vaginal misoprostol tablets (OR 4.4) were most likely to increase the risk of uterine hyperstimulation with FHR changes. Buccal/sublingual misoprostol (OR 4.25), oral misoprostol doses of 50 mcg or more (OR 2.85), vaginal misoprostol less than 50 mcg (OR 2.75), titrated low-dose oral misoprostol (OR1.93), and oral misoprostol less than 50 mcg (OR 1.13) also increased the risk of uterine hyperstimulation compared to placebo. There was insufficient data for the other safety outcomes to determine which interventions were safer.12
See Appendix 4, for further detail regarding SRs, and for RCTs.
Cost-Effectiveness of Misoprostol for Cervical Ripening and Induction of Labour
The Bierut economic model projected that for induction of labour, misoprostol vaginal inserts would cost less than dinoprostone vaginal tablets, gels, or inserts in four out of five European countries examined in the model. The savings was estimated from the time to delivery and time to active labour. Foley catheters were projected to cost less money than misoprostol vaginal inserts in Poland. However, the authors did not discuss the financial impact of implementing misoprostol vaginal inserts compared to the other interventions. A major limitation of the study was the limited number of responses to questionnaires about costs.24
For a hospital in the United Kingdom, economic modelling projected resource savings with vaginal misoprostol suppositories compared to dinoprostone vaginal inserts. The projected time to vaginal delivery with misoprostol vaginal inserts would be less, there would be fewer midwife shifts over one year, and it would also reduce the number of hours in the labour and delivery suite. The model also projected a 3.9 fold increase in the overall incidence of adverse effects for misoprostol compared with dinoprostone. The model did not consider the financial impact of using misoprostol vaginal inserts. Another possible limitation is extrapolating data from the EXPEDITE study conducted in the United States to a United Kingdom hospital. The applicability of the economic model in this study may also be limited by using cost data from one hospital in the UK.25
The Alfirevic meta-analysis and cost-effectiveness study12 concluded that buccal/sublingual misoprostol had the highest expected net benefit and highest probability of being coste-ffective compared to other interventions, and titrated low-dose oral misoprostol was the most cost-effective for the UK NHS; however, there was considerable uncertainly in the cost-effectiveness estimates. In women with intact membranes, the NHS intervention with the highest expected net benefit at any willingness-to-pay value was IV oxytocin with amniotomy, and buccal/sublingual misoprostol and titrated low-dose oral misoprostol had a moderate probability of being cost-effective; again, there was uncertainty in the cost estimate.12
The ten Eikelder cost-effectiveness study26 concluded that oral misoprostol and Foley catheter generated comparable costs in a Dutch hospital setting. There was also no significant difference between the interventions for composite risk of asphyxia and/or postpartum hemorrhage, nor for cesarean section rates.26
The Leigh economic trial23 concluded that in low-resource settings, like India, low-dose oral misoprostol 25 mcg used fewer resources than Foley catheters for labour induction. The mean treatment costs were lower in the misoprostol group than those receiving Foley catheters, and the costs of neonatal care were similar between the groups. The sensitivity analysis showed that oral misoprostol had a higher probability of being cost-saving over Foley catheter administration, and higher rates of labour induction, vaginal delivery, and vaginal delivery within 24 hours.23
See in Appendix 4 for details on these economic evaluations.
Guideline
The 2013 SOGC guidelines1 stated that misoprostol is a safe and effective agent for labour induction with intact membranes for inpatients (high level of evidence). The guidelines recommend avoiding misoprostol for vaginal birth after cesarean section, due to the increased risk of uterine rupture. (low level of evidence) The guidelines also recommend starting oxytocin no earlier than 4 hours after the last misoprostol dose (low level of evidence). The guideline stated that misoprostol is more effective than dinoprostone for achieving vaginal delivery but there is more uterine tachysystole. Misoprostol also reduces cesarean section rates, and rates are similar for both oral and vaginal routes. They stated that lower misoprostol vaginal doses of 25 mcg require more oxytocin, and that vaginal doses of 50 mcg or more cause more uterine tachysystole. The authors recommend electronic fetal monitoring for 30 minutes after misoprostol administration and for 60 minutes after any tachysystole episodes.1
See in Appendix 4 for details on this guideline.
Limitations
For clinical effectiveness, there were a wide variety of misoprostol dosage forms and doses in the systematic reviews and clinical trials, leading to a lack of generalizability in the results. There was also diversity in the primary and secondary outcomes, and many of the RCTs were too small to show significance for secondary outcomes like maternal or neonatal adverse effects, which is essential to determine patient monitoring practices. The obstetrical practices from the diverse countries in the trials and systematic review may not reflect practice in Canada, so the results may not be applicable to the Canadian patient population. Most trials did not report on patient satisfaction with methods of labour induction, which is an important consideration when implementing a practice change.
The economic models for misoprostol vaginal inserts were based on UK and European data from 5 other countries, so the cost data may not be applicable to the Canadian healthcare system.24,25 Some of the dosage forms included in these meta-analyses are not available in Canada, such as misoprostol vaginal inserts.
The lack of transparency in the 2013 SOGC guidelines make it difficult to assess whether all sources of information were included, and how the data was extracted. Also, the study was published in 2013, so newer data is not included in the guideline.1
Conclusions and Implications for Decision or Policy Making
There were two systematic reviews, none RCTs, five economic evaluations, and one evidence-based guideline that addressed the research questions. The 2013 SOGC guidelines stated that misoprostol is a safe and effective agent for labour induction with intact membranes for inpatients.1 This is confirmed with more recent data, as the Alfirevic meta-analyses concluded that misoprostol appears to be effective and safe for cervical ripening and induction of labour. This meta-analysis found that oxytocin with amniotomy and misoprostol were the most effective in achieving vaginal births relatively rapidly.12
For safety, based on the studies included in this review, the risk of uterine rupture with misoprostol use may be a concern, but there is conflicting data about the incidence. The rates from different studies for uterine rupture with prostaglandins range from 24.5 per 10001 to 9 per 1000.2 The latter prospective study showed no statistically difference in the rate of uterine rupture between prostaglandins and other methods of induction.2 Misoprostol is also considered a safe agent for labour induction by the World Health Organization (WHO). The 2011 WHO guidelines recommend misoprostol for the induction of labour, except in those with a previous cesarean section.30 Nonetheless, a risk versus benefit approach should be considered before implementing misoprostol at Canadian sites.
None of the included economic studies were done with Canadian data or for the Canadian healthcare setting. The Alfirevic cost-effectiveness study concluded that titrated low-dose and buccal/sublingual misoprostol had the highest probability of being the most cost-effective compared to other interventions, including vaginal and cervical dinoprostone, but this was based on UK National Health Service costs.
Cost-effectiveness studies with Canadian data are required to assess misoprostol oral and vaginal formulations with comparators currently used in Canada, such as dinoprostone vaginal inserts. Canadian data is also needed to address patient preferences for misoprostol use compared with other interventions. Updated Canadian guidelines are required to assess the more recent literature, to provide current dosing and administration information based on the various misoprostol formulations and doses found in the literature. Current guidelines are also needed to address implementation requirements of misoprostol for induction of labour in Canadian healthcare facilities. Finally, updated guidelines are required to assess patient safety data and monitoring requirements, to ensure safe outcomes for pregnant persons, fetuses, and neonates with misoprostol use in induction of labour.
- a
Recognizing that there are a range of gender expressions and identities, we are using the gender identities presented by study authors when reporting inclusion criteria and results.
References
- 1.
Leduc
D, Biringer
A, Lee
L, Dy
J, Clinical Practice Obstetrics C, Special C. Induction of labour.
Journal of Obstetrics & Gynaecology Canada: JOGC. 2013;35(9):840–857. [
PubMed: 24099451]
- 2.
Grobman
W. Cervical ripening and induction of labor in women with a prior cesarean delivery. In: Post
WW, ed. UpToDate. Waltham (MA): UpToDate; 2018. Accessed 2018 Nov 22.
- 3.
- 4.
- 5.
- 6.
Misoprostol: Drug information. In: Post
WW, ed. UpToDate. Waltham (MA): UpToDate; 2018. Accessed 2018 Nov 22.
- 7.
- 8.
- 9.
- 10.
- 11.
Liberati
A, Altman
DG, Tetzlaff
J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
- 12.
Alfirevic
Z, Keeney
E, Dowswell
T, et al. Methods to induce labour: a systematic review, network meta-analysis and cost-effectiveness analysis.
BJOG. 2016;123(9):1462–1470. [
PMC free article: PMC5021158] [
PubMed: 27001034]
- 13.
Ten Eikelder
ML, Mast
K, van der Velden
A, Bloemenkamp
KW, Mol
BW. Induction of Labor Using a Foley Catheter or Misoprostol: A Systematic Review and Meta-analysis.
Obstet Gynecol Surv. 2016;71(10):620–630. [
PubMed: 27770132]
- 14.
Conde
A, Ben
S, Tarigo
J, et al. Comparison between vaginal and sublingual misoprostol 50 micro g for cervical ripening prior to induction of labor: randomized clinical trial.
Arch Gynecol Obstet. 2017;295(4):839–844. [
PubMed: 28204882]
- 15.
Husain
S, Husain
S, Izhar
R. Oral misoprostol alone versus oral misoprostol and Foley’s catheter for induction of labor: A randomized controlled trial.
J Obstet Gynaecol Res. 2017;43(8):1270–1277. [
PubMed: 28561987]
- 16.
Mundle
S, Bracken
H, Khedikar
V, et al. Foley catheterisation versus oral misoprostol for induction of labour in hypertensive women in India (INFORM): a multicentre, open-label, randomised controlled trial.
Lancet. 2017;390(10095):669–680. [
PubMed: 28668289]
- 17.
Osoti
A, Kibii
DK, Tong
TMK, Maranga
I. Effect of extra-amniotic Foley’s catheter and vaginal misoprostol versus vaginal misoprostol alone on cervical ripening and induction of labor in Kenya, a randomized controlled trial.
BMC Pregnancy Childbirth. 2018;18(1):300. [
PMC free article: PMC6044072] [
PubMed: 30001195]
- 18.
Pimentel
VM, Arabkhazaeli
M, Moon
JY, et al. Induction of labor using one dose vs multiple doses of misoprostol: a randomized controlled trial.
Am J Obstet Gynecol. 2018;218(6):614.e611–614.e618. [
PubMed: 29614276]
- 19.
Pourali
L, Saghafi
N, Eslami Hasan Abadi
S, Tara
F, Vatanchi
AM, Motamedi
E. Induction of labour in term premature rupture of membranes; oxytocin versus sublingual misoprostol; a randomised clinical trial.
J Obstet Gynaecol. 2018;38(2):167–171. [
PubMed: 28784054]
- 20.
Rouzi
AA, Alsahly
N, Alamoudi
R, et al. Randomized clinical trial between hourly titrated and 2 hourly static oral misoprostol solution for induction of labor.
Am J Obstet Gynecol. 2017;216(4):405.e401–405.e406. [
PubMed: 27986461]
- 21.
Wang
X, Yang
A, Ma
Q, Li
X, Qin
L, He
T. Comparative study of titrated oral misoprostol solution and vaginal dinoprostone for labor induction at term pregnancy.
Arch Gynecol Obstet. 2016;294(3):495–503. [
PMC free article: PMC4981622] [
PubMed: 26746850]
- 22.
Yenuberi
H, Abraham
A, Sebastian
A, Benjamin
SJ, Jeyaseelan
V, Mathews
JE. A randomised double-blind placebo-controlled trial comparing stepwise oral misoprostol with vaginal misoprostol for induction of labour.
Trop Doct. 2016;46(4):198–205. [
PubMed: 26787644]
- 23.
Leigh
S, Granby
P, Haycox
A, et al. Foley catheter vs. oral misoprostol to induce labour among hypertensive women in India: a cost-consequence analysis alongside a clinical trial.
BJOG. 2018;21:21. [
PMC free article: PMC6282740] [
PubMed: 29782065]
- 24.
Bierut
A, Dowgiallo-Smolarczyk
J, Pieniazek
I, et al. Misoprostol Vaginal Insert in Labor Induction: A Cost-Consequences Model for 5 European Countries-An Economic Evaluation Supported with Literature Review and Retrospective Data Collection.
Adv Ther. 2016;33(10):1755–1770. [
PMC free article: PMC5055557] [
PubMed: 27549327]
- 25.
Draycott
T, van der Nelson
H, Montouchet
C, Ruff
L, Andersson
F. Reduction in resource use with the misoprostol vaginal insert vs the dinoprostone vaginal insert for labour induction: a model-based analysis from a United Kingdom healthcare perspective.
BMC Health Serv Res. 2016;16:49. [
PMC free article: PMC4750172] [
PubMed: 26864022]
- 26.
Ten Eikelder
M, van Baaren
GJ, Oude Rengerink
K, et al. Comparing induction of labour with oral misoprostol or Foley catheter at term: cost-effectiveness analysis of a randomised controlled multi-centre non-inferiority trial.
BJOG. 2018;125(3):375–383. [
PubMed: 28440898]
- 27.
Ten Eikelder
ML, Oude Rengerink
K, Jozwiak
M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial.
Lancet. 2016;387(10028):1619–1628. [
PubMed: 26850983]
- 28.
Nagdeve
P, Shetty
A. Review of use of Mysodelle as a method of induction of labour.
J Obstet Gynaecol. 2018;38(5):726. [
PubMed: 29944050]
- 29.
Duro-Gomez
J, Garrido-Oyarzun
MF, Rodriguez-Marin
AB, de la Torre Gonzalez
AJ, Arjona-Berral
JE, Castelo-Branco
C. What can we do to reduce the associated costs in induction of labour of intrauterine growth restriction foetuses at term? A cost-analysis study.
Arch Gynecol Obstet. 2017;296(3):483–488. [
PubMed: 28698953]
- 30.
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Systematic Reviews and Meta-Analyses
View in own window
| First Author, Publication Year, Country | Study Designs and Numbers of Primary Studies Included | Population Characteristicsa | Intervention and Comparator(s) | Clinical Outcomes, Length of Follow-Up |
|---|
| Alfirevic, 2016,12 United Kingdom | Included 611 RCTs (N = 103,041). | Women eligible for third-trimester induction of labour. | No treatment, placebo, and all pharmacological, mechanical, and complementary methods for induction of labour. Pharmacological comparators included misoprostol less than 50 mcg and 50 mcg or more (vaginal, oral, titrated oral low-dose, SR vaginal pessary, buccal, SL), PGE2 (vaginal, vaginal gel and SR pessary, intracervical), mifepristone, nitrous oxide, and IV oxytocin. Mechanical comparators included double-balloon or Cook’s catheter, and Foley catheter. | Key outcomes: Efficacy, safety and acceptability of induction method to women. Primary outcomes:
VD not achieved within 24 hours uterine hyperstimulation with FHR changes serious neonatal morbidity or perinatal death serious maternal morbidity or death instrumental delivery maternal satisfaction with IOL method cost, resource use, and utilities
Post-analysis outcomes:
|
| ten Eikelder, 2016,13 Netherlands | Included 22 RCTs (n=5,015) | Pregnant women scheduled for third trimester induction of labor, with an unfavorable cervix, a viable fetus in cephalic presentation, and no prior cesarean delivery. | Foley catheter compared with misoprostol (any dose, any route) for labor induction, or Foley catheter and misoprostol versus misoprostol. | Primary safety outcomes:
uterine hyperstimulation meconium stained liquor cesarean delivery for nonreassuring FHR vaginal instrumental delivery for nonreassuring FHR, postpartum hemorrhage arterial umbilical cord pH of less than 7.05 Apgar score of less than 7 NICU admission neonatal mortality
Primary outcomes for effectiveness of induction of labor:
total cesarean delivery rate, cesarean delivery for failure to progress first stage total vaginal instrumental deliveries
|
FHR = fetal heart rate; IOL = induction of labour; NICU = neonatal intensive care unit; PGE2 = prostaglandin E2 (dinoprostone); RCT = randomized controlled trial, SL = sublingual; SR = sustained release; VD = vaginal delivery;
- a
Recognizing that there are a range of gender expressions and identities, we are using the gender identities presented by study authors when reporting inclusion criteria and results.
Table 3Characteristics of Included Primary Clinical Studies
View in own window
| First Author, Publication Year, Country | Study Design | Population Characteristicsa | Intervention and Comparator(s) | Clinical Outcomes, Length of Follow-Up |
|---|
| Osoti, 2018,17 Kenya | Open-label randomized trial | N = 180 Pregnant women at gestational age 28 weeks or more. Inclusion criteria:
indications for IOL (late-term or post-term pregnancies, preeclampsia, chronic hypertension, gestational diabetes, oligohydramnios, or intrauterine fetal demise) Bishop score less than 6 singleton cephalic pregnancy intact membranes no contraindications to vaginal delivery.
Exclusion criteria:
favourable Bishop score fetal growth restriction previous CS estimated fetal weight more than 4 kg placenta previa non-reassuring fetal status grand multiparity HIV infection uncertain gestational age
| Vaginal misoprostol 25 mcg every 6 hours, up to a maximum of 4 doses.
Extra-amniotic Foley catheter plus vaginal misoprostol 25 mcg every 6 hours, up to a maximum of 6 doses.
| Primary outcome: Incidence of failed induction (inability to achieve cervical dilatation of more than 3 cm 24 h after induction). Secondary outcomes:
induction-to-delivery time adverse maternal outcomes (postpartum hemorrhage, uterine rupture) adverse perinatal outcomes (poor Apgar, NICU admission, nonreassuring fetal status)
|
| Pimentel, 2018,18 United States | Open-label randomized trial | N = 243 Pregnant women aged 18 to 50 years. Inclusion criteria:
Exclusion criteria:
contraindication to misoprostol or vaginal delivery fetal death major fetal anomaly fetal growth restriction nonreassuring antepartum fetal testing premature rupture of membranes
| Vaginal misoprostol 25 mcg for 1 dose.
Vaginal misoprostol 25 mcg every 4 to 6 hours, for a maximum of 4 doses.
| Primary outcome: Vaginal delivery within 24 hours (from time of first misoprostol dose to vaginal delivery). Secondary outcomes:
vaginal delivery within 12 hours delivery within 24 hours regardless of mode time to vaginal delivery time to delivery regardless of mode total vaginal and CS delivery rates indications for and factors associated with CS cervical dilation before CS Bishop score before the start of oxytocin, rate of oxytocin use
Maternal secondary outcomes
rates of chorioamnionitis, endometritis PPH need for blood transfusion third- and fourth degree lacerations, ICU admission Death
Neonatal outcomes:
|
| Pourali, 2018,19 Iran | Open-label randomized trial | N = 270 Pregnant women with PROM between 37 and 42 weeks of gestation, and:
singleton pregnancy cephalic presentation history of <5 vaginal deliveries no hypersensitivity to prostaglandins no contraindication to vaginal delivery no other cervical ripening methods within 7 days of hospitalization no previous uterine scar, Bishop scores of 6 or less (unripe cervix) reassuring fetal heart rate patterns
Exclusion criteria:
more than three uterine contractions in 10 minutes abnormal fetal heart rate patterns potential cephalopelvic disproportion patient’s refusal for being in the study. Women requiring oxytocin infusion after receiving misoprostol
| Intervention: Misoprostol 25 mcg sublingual every 4 hours for a total of 6 doses. Comparator: Oyxtocin IV starting at 2 mU/minute and increasing by 2 mU/minute every 20 minutes until adequate contractions (4 to 5 contractions every 10 minutes). | Primary outcome was the mean time between the start of labour induction and the start of active labour. Secondary outcomes were duration of active labour, duration of second stage of labour, and side-effects of misoprostol and oxytocin. |
| Conde, 2017,14 Uruguay | Single-blind randomized trial | N=102 Pregnant women with:
gestational ages between 32/0 and 41/6, viable fetus in cephalic presentation estimated fetal weight less than 4 kg no contraindication for vaginal birth
| A single dose of vaginal misoprostol 50 mcg compared with a single dose of sublingual misoprostol 50 mcg. | Primary outcome: Variation of at least 2 points of the Bishop score at 6 hours after misoprostol administration. Secondary outcomes:
Tachysystole Frequency of vaginal birth and cesarean section Frequency for new doses needed to continue IOL Frequency of labour diagnosis 6 hours after misoprostol administration
|
| Husain, 2017,15 Pakistan | Open-label randomized trial | N = 335 Inclusion criteria: Pregnant women aged 20 to 40 years, with a singleton pregnancy and a fetus in cephalic presentation as assessed by ultrasound at term (i.e., gestational age ≥ 37 weeks by last menstrual period and dating scan). Exclusion criteria:
Bishop score of > 4, cephalopelvic disproportion on exam history of placenta previa or unexplained vaginal bleeding, history of previous CS or other uterine surgery, active herpes simplex infection suspected on exam and history chorioamnionitis suspected on history and exam any contraindication to use of prostaglandins acute pelvic inflammatory disease, any contraindication to vaginal delivery nonreassuring fetal heart rate pattern prior to induction
| Intervention: Oral misoprostol 50 mcg (every 4 hours for a maximum of 4 doses) plus intracervical Foley catheters. Oxytocin started when uterine contractions less than 3/minute. Comparator: Oral misoprostol 50 mcg alone, administered every 4 hours for a maximum of 4 doses. Oxytocin started when uterine contractions less than 3/minute. | Primary outcome: Rate of failure to achieve vaginal delivery within 24 hours of labour induction with either intervention. Secondary outcomes:
|
| Mundle, 2017,16 India | Multicentre, open-label randomized trial | N = 602 Pregnant women 18 years of age or older Inclusion criteria:
Exclusion criteria:
| Transcervical Foley catheters:
Remained in place until expelled with active labour or until 12 hours had elapsed. If expelled within 12 hours without active labour, or if 12 hours elapsed with no labour, membranes were ruptured and oxytocin was given.
Oral misoprostol 25 mcg:
| Primary outcome:
Secondary outcomes:
induction to birth interval (vag, CS, and all births) vaginal births within 12 hours cervix unchanged at 12 hours and 24 hours, oxytocin augmentation, time from randomisation to start of induction and birth total doses of misoprostol number of participants given a 50 mcg dose maternal complications fetal or neonatal complications women’s expectations about labour
|
| Rouzi, 2017,20 Saudi Arabia | Open-label randomized trial | N = 146 Pregnant women indicated for IOL: Inclusion criteria:
Exclusion criteria:
hypersensitivity to misoprostol previous cesarean delivery or uterine surgery severe pregnancy-induced hypertension total pregnancies of 4 or more uterine contractions significant maternal cardiac, renal, or liver disease
| Oral misoprostol 1 mcg/mL solution, administered hourly
started with 20 mcg/hour administered for 4 doses or less in absence of uterine activity, the doses were increased to 40 mcg/hour for 4 doses or less, and then to 60 mcg/hour for 16 doses or less.
Compared with oral misoprostol 25 mcg dose (1 mcg/mL oral solution) administered every 2 hours for a total of 12 doses or until the onset of regular uterine activity. If contractions were inadequate, oxytocin was started at least 2 hours after the last misoprostol dose. | The primary outcome was vaginal delivery within 24 hours after treatment initiation. Secondary outcomes were rate of cesarean delivery and oxytocin augmentation requirements. Safety outcomes included incidence of maternal morbidity and adverse neonatal outcomes. |
| Wang, 2016,21 China | Multicentre openlabel randomized trial | N = 411 Pregnant women undergoing IOL. Inclusion criteria:
singleton pregnancies with occipital presentation nullipara gestational age is at least 36 weeks Bishop score less than six no vaginal delivery contraindication (e.g. cephalopelvic disproportion, mal-presentation, fetal compromise, nonreassuring FHR pattern, previous scar and antepartum hemorrhage)
Exclusion criteria:
contraindication to induction and vaginal delivery prostaglandin allergy glaucoma, asthma, allergic colitis cardiac, hepatic, renal, or adrenal cortex insufficiency
| Intervention: Misoprostol 1 mg/mL oral solution:
20 mcg hourly for 2 doses 30 mcg hourly for 3 doses 40 mcg administered 90 minutes later 50 mcg administered 2 hours later 60 mcg every 2 hours for 2 doses
Comparator:Vaginal dinoprostone |
Labour induction Mode of delivery Induction-to-delivery time Bishop score Total misoprostol dose Oxytocin use Maternal morbidity Fetal tachycardia Meconium-stained liquor Apgar scores <7 at 1, 5, and 10 minutes
|
| Yenuberi, 2016,22 India | Double-blind RCT | N = 778 Inclusion criteria: Women to benefit from IOL with:
37 and 42 weeks gestation singleton live fetus in cephalic presentation Bishop score <6, intact membranes reassuring fetal cardiotocograph tracing
Exclusion criteria:
| Intervention: Oral misoprostol every 4 hours for 3 doses:
Vaginal placebo every 4 hours for 3 doses.Comparator: Vaginal misoprostol 25 mcg every 4 hours for 3 doses. Oral placebo every 4 hours for 3 doses.
| Primary outcomes:
Vaginal delivery within 24h of induction Bishop score at amniotomy Duration from IOL start to delivery Vaginal delivery in 12 hours Delivery by lower segment CS Oxytocin augmentation Total number of misoprostol doses
|
CS = cesarean section; ICU = intensive care unit; IOL = induction of labour; kg = kilogram; mU = milliunits; NICU = neonatal intensive care unit; PPH = postpartum hemorrhage; PROM = premature rupture of membranes; RCT = randomized controlled trial
- a
Recognizing that there are a range of gender expressions and identities, we are using the gender identities presented by study authors when reporting inclusion criteria and results.
Table 4Characteristics of Included Economic Evaluations
View in own window
| First Author, Publication Year, Country | Type of Analysis, Time Horizon, Perspective | Decision Problem | Population Characteristics | Intervention and Comparator (s) | Approach | Clinical and Cost Data Used in Analysis | Main Assumptions |
|---|
| Leigh, 201823, UK | Cost-consequence analysis, short-term, Indian healthcare system | Relative cost-effectiveness of misoprostol and Foley catheter for IOL in women with gestational hypertension in low resource settings. | Women requiring delivery for hypertension or pre-eclampsia (Mundle et al., 2017) | Transcervical Foley catheters:
Remained in place until expelled with active labour or until 12 hours had elapsed. If expelled within 12 hours without active labour, or if 12 hours elapsed with no labour, membranes were ruptured and oxytocin was given.
Oral misoprostol 25 mcg:
| Trial-based analysis | Clinical data from the 2017 Mundle trial16 Cost data from an Indian medical college, and patient costs collected at bedside by trial administrators. |
Vaginal delivery rate of 41% with Foley catheter Midwives administer misoprostol at 2 hours intervals Financial costs for staff time equivalent
|
| ten Eikelder, 2018,26 Netherlands | Cost-effectiveness analysis, short term, hospital perspective | The economic consequences of labour induction with oral misoprostol compare to Foley catheter. | Women with a viable singleton pregnancy in cephalic presentation, intact membranes, a gestational age of 37 weeks or more, no previous caesarean section, Bishop score <6, scheduled for IOL | Oral misoprostol 50 mcg every 4 hours (total 3 doses in 24 hours) compared with Foley catheter. | Trial-based analysis | Clinical data from 1 multicenter trial (n = 1,845). Resource costs were collected from case record forms, medication costs from the Dutch Pharmacother apeutic Compass, and cost of induction methods from a university medical centre hospital pharmacist. | The duration of labour ward occupation was calculated as the interval between admission to the labour ward and birth, with the addition of 1 hour for recovery care. Costs of a caesarean section were estimated as the costs of 1 hour in the operating theatre. |
| Alfirevic, 2016,12 UK | Decision-analytic model, short-term, UK perspective | Cost-effectiveness of different IOL methods | Pregnant women carrying a viable fetus who were eligible for any method of third trimester labour induction | Interventions
Vaginal misoprostol < 50 mcg Vaginal misoprostol ≥ 50 mcg Oral misoprostol < 50 mcg Oral misoprostol ≥ 50 mcg. Titrated lowdose oral misoprostol SR misoprostol insert Buccal/ sublingual misoprostol Vaginal PGE2 tablet. Vaginal PGE2 gel Vaginal PGE2 SR pessary Intracervical PGE2 Vaginal PGE2 NR pessary IV oxytocin IV oxytocin with amniotomy NO Mifepristone. Foley catheter. Double-balloon or Cook’s catheter Extra-amniotic PGE2. Placebo
| Trial-based analysis. | Clinical data from meta-analysis. Costs were average UK NHS costs for intervention, method of delivery, and length of neonatal stay in various care-level units. Price year was 2012-13. |
Relative effects for NICU admission independent of delivery timing but dependent on delivery mode Probability of NICU admission 1.5 times higher for CS VD within 24 hours was short stay, and more than 24 hours (including CS) was long stay, with uniform distribution of these costs Vaginal PGE2 NR pessary cost equal to tablet and gel Most cost differences between IOL methods occur during and immediately after birth LOS in ICU = 2 days, 1.5 days in highdependency care, 2 days in transitional care Instrumental delivery, Apgar < 7 at 5 minutes, and uterine hyperstimulation not reported and would be captured in other outcomes
|
| Bierut, 2016,24 Poland | Cost-consequences model with macro costing, no time horizon indicated, European perspective. | Compare the total healthcare IOL costs in Austria, Poland, Romania, Russia, and Slovakia using MVI with relevant alternatives. | Pregnant women undergoing induction of labour. | Intervention: Misoprostol vaginal insert (MVI) Comparators: Dinoprostone vaginal insert, dinoprostone cervical gel, oxytocin, and Foley or Cook catheters. | Trial-based analysis. | Clinical data: Meta-analysis of 11 RCTs for time to delivery and time to active labour. Cost data: Retrospective real-world units costs and resource utilization from the 5 countries, from a questionnaire. |
Patient spends time before active labor within the antenatal ward and then enters the labor ward for labor. The IOL can end with vaginal delivery (successful) or with cesarean delivery (failure). After delivery, patient and neonate are in postnatal ward until discharge.
|
| Draycott, 2016,25 United Kingdom | Markov model-based analysis, short-term, UK NHS perspective | To estimate and compare healthcare resource used associated with labour induction using MVI instead of DVI | Pregnant women undergoing induction of labour. | Intervention: Misoprostol vaginal insert Comparator: Dinoprostone vaginal insert. | Trial-based analysis. | Clinical data: Efficacy and safety data from EXPEDITE study (published and non-published data).
Cost data: Clinical data, parity distribution, and resource use from Southmead Hospital in Bristol, UK. |
Cycle length of 1 hour. Different IOL methods were mutually exclusive. All women received only 1 of the comparators. Onset of labour and delivery were a function of the time since induction. Aside from Southmead, other demographics assumed to be identical to EXPEDITE population.
|
CS = cesarean section; DVI = dinoprostone vaginal insert; IOL = Induction of labour; NHS = National Health Service; NR = normal release; MVI = misoprostol vaginal insert; NO = nitrous oxide; PGE2 = dinoprostone; SR = slow release; RCT = randomized controlled trial, UK = United Kingdom; VD = vaginal delivery
Table 5Characteristics of Included Guideline
View in own window
| Intended Users, Target Population | Intervention and Practice Considered | Major Outcomes Considered | Evidence Collection, Selection, and Synthesis | Evidence Quality Assessment | Recommendations Development and Evaluation | Guideline Validation |
|---|
| Leduc, 20131 |
|---|
Intended users: obstetrical care providers Target population: pregnant womena | Induction of labour | Appropriate time and methods of induction, appropriate mode of delivery, and optimal maternal and perinatal outcomes. | Electronic databases (Pubmed, CINAHL, Cochrane Library) searched and updated to December 2010, and searching of grey literature. No information on selection or synthesis. | Evidence rated using ranking system adapted from the CTFPHC. Quality of evidence: I: At least 1 properly randomized CT. II-1: NR well-designed controlled trials. II-2: Well-designed prospective or retrospective cohort or case-control studies, preferably from more than 1 centre or research group. II-3: Comparisons between times or places with/wihout the intervention. Dramatic results in uncontrolled experiments could also be included. III: Opinion of respected authorities (from clinical experience, descriptive studies, or expert committee reports). Classification of recommendations: A: Good evidence to recommend the clinical preventive action. B. Fair evidence to recommend the clinical preventive action. C: Conflicting existing evidence which does not allow recommendation for or against the clinical preventive action. Other factors may influence decision-making. D. Good evidence to recommend against the clinical preventive action. L. There is insufficient evidence (quantity or quality) to make a recommendation. Other factors may influence decision-making. | Evidence rated according to the adapted CTFPHC system. Not stated how consensus was reached. | Not stated. |
CT = controlled trial; CTFPHC = Canadian Task Force on Preventive Health Care; NR = nonrandomized; RCT = randomized controlled trial
Appendix 3. Critical Appraisal of Included Publications
Table 6Strengths and Limitations of Systematic Reviews and Meta-Analyses using AMSTAR8
View in own window
| Strengths | Limitations |
|---|
| Alfirevic et al., 201612 |
|---|
PICO format followed. Review methods were established prior to the review, including the review questions, search strategy, inclusion and exclusion criteria, risk of bias assessment, plan for network meta-analysis, and assessment of heterogeneity. Search done for RCTs in all languages in Medline, Embase, Cochrane Library, CINAHL, trials registry portals, hand search of journal and conference proceedings, and selected journals not included in other databases. Study selection and data extraction done in duplicate. Had list of excluded studies and rationale for exclusion. List of included studies had populations, interventions and doses, comparators and doses, outcomes, research designs, and funding sources. Risk of bias assessed for RCTs based on allocation concealment and blinding of assessors. Authors consulted the Cochrane Handbook for Systematic Reviews (v. 5.1.0).for risk of bias, and excluded studies of unclear or high risk of bias. Network meta-analysis performed within Bayesian framework using OpenBUGS (v. 3.2.3), and also pairwise metaanalyses for intervention comparators when possible, and discussed heterogeneity in results.
|
No explanation for inclusion of only RCTs. Exclusion of other studies could lead to bias. Did not search the reference lists and bibliographies of the included studies, which may have excluded studies that met criteria. List of included studies did not include timeframe for follow-up. Authors did not report on publication bias, so no sensitivity analyses on missing “null” studies, which could potentially invalidate results. Authors did not report own competing interests, which could conceal bias.
|
| ten Eikelder et al., 201613 |
|---|
Research question had PICO elements. Review methods were established prior to the review, including the review questions, search strategy, inclusion and exclusion criteria, risk of bias assessment, plan for network meta-analysis, and assessment of heterogeneity. Search done for RCTs in all languages in Cochrane Library, Pubmed, Embase, and Web of Science. Study selection and data extraction done in duplicate. Risk of bias assessed for RCTs according to the Cochrane Handbook for Systematic Reviews of Interventions (v.5.1.0), and excluded studies with high risk of bias. Meta-analysis performed using RevManversion 5.3, and each meta-analyses assessed for heterogeneity using the fixed-effect model, and the random-effects model for significant heterogeneity. Authors discussed effects of heterogeneity of study design PICO elements on the results. Authors did not have any competing interests.
|
Unknown whether literature search restricted to language, so unable to determine whether eligible studies were excluded. No explanation for inclusion of only RCTs. Exclusion of other studies could lead to bias. Performed subgroup analyses for different doses of misoprostol, which were not included in primary outcomes. Did not search the reference lists and bibliographies of the included studies, which may have excluded studies that met criteria. Did not search trial/study registries, which may have excluded studies that met criteria. Did not provide a list of excluded studies; unjustified exclusion may bias the review findings. The included trials were not described in detail (PICO, research designs), which does not permit user to evaluate whether trial inclusion was appropriate, nor permit examination for heterogeneity of intervention effects. Did not include funding sources for included studies, which could be a source of bias.
|
Table 7Strengths and Limitations of Clinical Studies using Cochrane risk-of-bias tool (RoB 2.0)9
View in own window
| Strengths | Limitations |
|---|
| Osoti et al., 201817 |
|---|
|
|
Risk of bias due to assignments to the intended intervention and deviations from interventions – some concerns:
Study participants and caregivers were not blinded to the intervention. Participants did adhere to the assigned intervention. Unknown if both groups had balanced cointervention (amniotomy + oxytocin), which could have affected outcomes.
Risk of bias in selection of the reported result – some concerns:
The trial was analyzed as per the pre-specified plan. However, additional statistics were reported that were not part of the primary or secondary outcomes.
|
| Pimentel et al., 201818 |
|---|
Risk of bias from the randomization process was low:
Risk of bias from missing outcome data was low:
Risk of bias in selection of the reported result was low:
|
|
| Pourali et al., 201819 |
|---|
Risk of bias in measuring outcomes was low:
|
|
| Conde et al., 201714 |
|---|
|
|
Risk of bias due to assignments to the intended intervention – some concerns:
Although the participants were blinded, the technician delivering the intervention was not blinded. The authors did perform an ITT analysis.
Risk of bias due to deviations from adherence to the intended intervention – some concerns:
Although the participants were blinded, and adhered to the intervention, the technician delivering the intervention was not blinded. Did not indicate if co-interventions (oxytocin) administered.
Risk of bias in measuring outcomes – some concerns:
The measurements were appropriate (e.g. Bishop score). However, the authors did not indicate if assessors of Bishop score after misoprostol administration knew intervention the patient received.
|
| Husain et al., 201715 |
|---|
|
|
| Mundle et al., 201716 |
|---|
|
|
Risk of bias due to assignments to the intended intervention and deviations from interventions – some concerns:
Study participants and caregivers were not blinded to the intervention. Participants did adhere to the assigned intervention. There was an ITT analysis.
|
| Rouzi et al., 201720 |
|---|
|
|
Risk of bias due to assignments to the intended intervention and deviations from interventions – some concerns:
Study participants and caregivers were not blinded to the intervention. Participants did adhere to the assigned intervention. There was an ITT analysis.
|
| Wang et al., 201621 |
|---|
|
|
Risk of bias due to assignments to the intended intervention and deviations from interventions – some concerns:
Study participants and caregivers were not blinded to the intervention. Participants did adhere to the assigned intervention. There was no ITT analysis for randomized patients excluded from the study
|
| Yenuberi et al., 201622 |
|---|
|
|
Risk of bias from missing outcome data – some concerns:
|
Table 8Strengths and Limitations of Guidelines using AGREE II10
View in own window
| Item | Guideline |
|---|
| SOGC, 20131 |
|---|
| Domain 1: Scope and Purpose |
|---|
| 1. The overall objective of the guideline is specifically described. | Yes |
| 2. The health questions covered by the guideline are specifically described. | Yes |
| 3. The population (patients, public, etc.) to whom the guideline is meant to apply is specifically described. | Yes |
| Domain 2: Stakeholder Involvement |
|---|
| 4. The guideline development group includes individuals from all relevant professional groups. | Unknown |
| 5. The views and preferences of the target population (patients, public, etc.) have been sought. | Unknown |
| 6. The target users of the guideline are clearly defined. | Yes |
| Domain 3: Rigour of Development |
|---|
| 7. Systematic methods were used to search for evidence. | Yes |
| 8. The criteria for selecting the evidence are clearly described. | No |
| 9. The strengths and limitations of the body of evidence are clearly described. | Yes |
| 10. The methods for formulating the recommendations are clearly described. | No |
| 11. The health benefits, side effects, and risks have been considered in formulating the recommendations. | Yes |
| 12. There is an explicit link between the recommendations and the supporting evidence. | Yes |
| 13. The guideline has been externally reviewed by experts prior to its publication. | Unknown |
| 14. A procedure for updating the guideline is provided. | No |
| Domain 4: Clarity of Presentation |
|---|
| 15. The recommendations are specific and unambiguous. | Yes |
| 16. The different options for management of the condition or health issue are clearly presented. | Yes |
| 17. Key recommendations are easily identifiable. | Yes |
| Domain 5: Applicability |
|---|
| 18. The guideline describes facilitators and barriers to its application. | No |
| 19. The guideline provides advice and/or tools on how the recommendations can be put into practice. | No |
| 20. The potential resource implications of applying the recommendations have been considered. | No |
| 21. The guideline presents monitoring and/or auditing criteria. | No |
| Domain 6: Editorial Independence |
|---|
| 22. The views of the funding body have not influenced the content of the guideline. | Unknown |
| 23. Competing interests of guideline development group members have been recorded and addressed. | No |
Table 9Strengths and Limitations of Economic Studies using the Drummond Checklist9
View in own window
| Strengths | Limitations |
|---|
| Leigh et al., 201823 |
|---|
The research question was stated, and all the interventions were described. The economic importance of the research question was stated, and the viewpoints were also clearly stated and justified. The economic model and details on key parameters were recorded, and its use justified, and the main assumptions were stated. The sources of effectiveness estimates were stated. The details and results of the effectiveness study were included. The primary economic outcomes and methods to value benefits were clearly stated. Methods for estimation of quantities and unit costs were described. Currency and price data was recorded, including adjustments for inflation and currency conversion. The time horizon of costs and benefits was stated. The sensitivity analysis was explained and the choice and range of variables was justified. Relevant alternatives were compared. The study question was answered. The conclusions followed the reported data, and were accompanied by the strengths and weaknesses.
|
|
| ten Eikelder et al., 201826 |
|---|
The research question was stated, and all the interventions were described. The economic importance of the research question was stated, and the viewpoints were also clearly stated and justified. The economic model and details on key parameters were recorded, and its use justified, and the main assumptions were stated. The sources of effectiveness estimates were stated. The details and results of the effectiveness study were included. The primary economic outcomes and methods to value benefits were clearly stated. Methods for estimation of quantities and unit costs were described. Currency and price data was recorded, including adjustments for inflation. The time horizon of costs and benefits was stated, and why discount rate was not included. The sensitivity analysis was explained and the choice and range of variables was justified. Relevant alternatives were compared. The incremental analysis was reported. The study question was answered. The conclusions followed the reported data, and were accompanied by the strengths and weaknesses.
|
Did not include discount rates, although all costs occurred within a year. Costs did not address maternal adverse outcomes directly with either comparator, nor were neonatal outcomes addressed.
|
| Alfirevic et al., 201612 |
|---|
The research question was stated, and all the interventions were described. The economic importance of the research question was stated, and the viewpoints were also clearly stated and justified. The economic model and details on key parameters were recorded, and its use justified, and the main assumptions were stated. The sources of effectiveness estimates were stated. The details and results of the effectiveness study were included. The primary economic outcomes and methods to value benefits were clearly stated. Methods for estimation of quantities and unit costs were described. Currency and price data was recorded. The time horizon of costs and benefits was stated, and why discount rate was not included. The sensitivity analysis was explained and the choice and range of variables was justified. Relevant alternatives were compared. The incremental analysis was reported. The study question was answered. The conclusions followed the reported data, and were accompanied by the strengths and weaknesses.
|
|
| Bierut et al., 201624 |
|---|
The research question was stated, and the alternatives to the main comparator (misoprostol) were described. The sources of effectiveness estimates used were stated. Some details of the meta-analysis of estimates were provided. A cost-consequences model was used for the economic evaluation, and the main assumptions were stated. The methods to estimate costs were described (macrocosting method). The price data for hospital costs, wages, and adverse events was recorded in Euros. The economic model and details on key parameters were recorded.
|
The economic importance of the research question and the analysis viewpoints were not stated. The details of the meta-analysis subjects were not stated. The quantities of resource use were not reported separately. No details were provided on reasons for the choice of economic model. The price data was not adjusted for inflation. The time horizon of the costs and benefits was not stated. There was no sensitivity or incremental analyses. Major outcomes were only presented in an aggregated form. Authors did not explain statistical significance in conclusions, nor how they arrived at that calculation. Conclusions follow from the data reported, but not all of the data was discussed. The study was paid for by Ferring Pharmaceuticals (manufacturer of the misoprostol vaginal insert), and the main author was an employee of Ferring Pharmaceuticals; this is a potential source of bias.
|
| Draycott et al., 201625 |
|---|
The research question was stated, and the alternative to misoprostol was described. The economic importance of the research question was stated, and the viewpoints were also clearly stated and justified. The economic model and details on key parameters were recorded, and authors justified its use, and the main assumptions were stated. The sources of effectiveness estimates were stated. The details and results of the effectiveness study were included. The primary economic outcomes and methods to value benefits were clearly stated. Productivity changes were reported and their relevance was discussed. The time horizon of costs and benefits was stated. The sensitivity analysis was explained and the choice and range of variables was justified. Relevant alternatives were compared. The study question was answered. The conclusions followed the reported data, and were accompanied by the strengths and weaknesses.
|
Did not provide details on those who provided economic valutions. There was no incremental analysis. The study was paid for by Ferring Pharmaceuticals (manufacturer of the misoprostol vaginal insert), and one of secondary authors was an employee of Ferring Pharmaceuticals; this is a potential source of bias.
|
Appendix 4. Main Study Findings and Authors’ Conclusions
Table 10Summary of Findings Included Systematic Reviews and Meta-Analyses
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Alfirevic et al., 201612 |
|---|
Failure to achieve VD within 24 hours compared to placebo (n = 28,845) An OR less than 1 favors the intervention. | “The active interventions most likely to achieve VD [vaginal delivery] within 24 hours were i.v. oxytocin with amniotomy, misoprosotol (vaginal tablets – high and low dose; pessary – sustained release; low-dose oral solution; and buccal/sublingual misoprotol) closely followed by vaginal administration of PGE2 (pessary – normal release). It should be stressed that the rankings have wide Crls for all of the above methods, indicating considerable uncertainty." p.105 |
| Intervention vs. Placebo | NMA OR | NMA 95% Crl |
|---|
| Oxytocin IV with amniotomy | 0.05 | 0.07 to 0.32 |
| Misoprostol vaginal 50 mcg or more | 0.09 | 0.06 to 0.24 |
| Misoprostol oral, titrated low-dose | 0.1 | 0.07 to 0.29 |
| Misoprostol vaginal less than 50 mcg | 0.11 | 0.09 to 0.32 |
| Misoprostol SR vaginal pessary | 0.11 | 0.05 to 0.22 |
| Misoprostol buccal/sublingual | 0.11 | 0.05 to 0.19 |
| Dinoprostone vaginal NR pessary | 0.11 | 0.05 to 0.19 |
| Dinoprostone vaginal gel | 0.13 | 0.08 to 0.5 |
| Dinoprostone vaginal SR pessary | 0.15 | 0.08 to 0.29 |
| Misoprostol oral 50 mcg or more | 0.16 | 0.05 to 0.2 |
| Dinoprostone vaginal tablet | 0.16 | 0.03 to 0.26 |
| Dinoprostone intracervical | 0.18 | 0.09 to 0.38 |
| Double-balloon or Cook’s catheter | 0.18 | 0.01 to 0.16 |
| Foley catheter | 0.19 | 0.09 to 0.46 |
| Oxytocin IV | 0.2 | 0.21 to 1.97 |
| Oral misoprostol less than 50 mcg | 0.22 | 0.07 to 0.39 |
| Dinoprostone extra-amniotic | 0.41 | 0.07 to 1.33 |
Cesarean section compared to placebo (n = 96,771) An OR less than 1 favors the intervention. | “Compared with placebo, several treatments showed a statistically significant reduction in the odds of CS [cesarean section]: titrated low-dose misoprostol, vaginal misoprostol at both≥ 50 μg and <50 μg, vaginal PGE2 [dinoprostone] gel, intracervical PGE2, oral misoprostol tablet (≥ 50 μg), Foley catheter, membrane sweeping and buccal/sublingual misoprostol. In this group, titrated oral misoprostol achieved the lowest odds of an eventual CS but there was still considerable uncertainty in this finding, as observed by the posterior mean rank of 6th (out of 33) and 95% Crl from 2nd to 13th (out of 33) for oral misoprostol solution." p. 105 |
| Intervention vs. Placebo | NMA OR | NMA 95% Crl |
|---|
| Misoprostol oral, titrated low-dose | 0.62 | 0.47 to 0.80 |
| Misoprostol buccal/ sublingual | 0.68 | 0.51 to 0.89 |
| Misoprostol vaginal less than 50 mcg | 0.7 | 0.57 to 0.85 |
| Misoprostol oral 50 mcg or more | 0.72 | 0.58 to 0.88 |
| Misoprostol vaginal 50 mcg or more | 0.73 | 0.08 to 2.59 |
| Membrane sweeping | 0.74 | 0.08 to 2.59 |
| Foley catheter | 0.76 | 0.61 to 0.95 |
| Dinoprostone vaginal gel | 0.79 | 0.61 to 0.95 |
| Laminaria | 0.8 | 0.61 to 0.95 |
| Dinoprostone vaginal SR pessary | 0.82 | 0.61 to 0.95 |
| Dinoprostone intracervical | 0.83 | 0.61 to 0.95 |
| Oxytocin IV with amniotomy | 0.89 | 0.61 to 0.95 |
| Oxytocin IV | 0.93 | 0.61 to 0.95 |
| Dinoprostone vaginal NR pessary | 0.89 | 0.61 to 0.95 |
| Misoprostol SR vaginal pessary | 0.98 | 0.61 to 0.95 |
| Dinoprostone extra-amniotic | 0.98 | 0.61 to 0.95 |
| Dinoprostone vaginal tablet | 1.04 | 0.61 to 0.95 |
| Amniotomy | 1.06 | 0.61 to 0.95 |
| Double-balloon or Cook’s catheter | 1.11 | 0.61 to 0.95 |
| Oral misoprostol less than 50 mcg | 1.11 | 0.61 to 0.95 |
Instrumental delivery compared to placebo (n = 54,511) An OR less than 1 favors the intervention. | “Using placebo as the reference intervention two interventions resulted in significant reduction in instrumental delivery, namely vaginal PGE2 [dinoprostone] pessary (slow release) and Foley catheter." p.41 |
| Intervention vs. Placebo | NMA OR | NMA 95% Crl |
|---|
| Foley catheter | 0.68 | 0.5 to 0.91 |
| Misoprostol buccal/ sublingual | 0.69 | 0.44 to 1.03 |
| Dinoprostone vaginal SR pessary | 0.72 | 0.50 to 0.99 |
| Misoprostol oral less than 50 mcg | 0.74 | 0.34 to 1.38 |
| Double-balloon or Cook’s catheter | 0.75 | 0.47 to 1.14 |
| Misoprostol vaginal less than 50 mcg | 0.8 | 0.59 to 1.05 |
| Laminaria | 0.83 | 0.47 to 1.38 |
| Misoprostol oral 50 mcg or more | 0.84 | 0.63 to 1.09 |
| Amniotomy | 0.86 | 0.5 to 1.38 |
| Dinoprostone intracervical | 0.89 | 0.5 to 1.38 |
| Dinoprostone vaginal tablet | 0.91 | 0.67 to 1.22 |
| Dinoprostone extra-amniotic | 0.91 | 0.49 to 1.52 |
| Misoprostol vaginal 50 mcg or more | 0.92 | 0.7 to 1.18 |
| Dinoprostone vaginal gel | 0.93 | 0.72 to 1.18 |
| Misoprostol SR vaginal pessary | 0.93 | 0.46 to 1.71 |
| Oxytocin IV with amniotomy | 0.93 | 0.64 to 1.31 |
| Misoprostol oral, titrated low-dose | 1 | 0.62 to 1.52 |
| Dinoprostone vaginal NR pessary | 1.08 | 0.79 to 1.45 |
| Oxytocin IV | 1.08 | 0.83 to 1.39 |
| Membrane sweeping | 1.2 | 0.84 to 1.66 |
Uterine hyperstimulation compared to placebo (n-43,612) An OR less than 1 favors the intervention. | Uterine hyperstimulation with FHR [fetal heart rate] changes was one of the key safety outcomes. Here double-balloon catheter, NO [nitric oxide] and laminaria had the highest probability of being among the best three treatments, whereas i.v. oxytocin with amniotomy, slow-release misoprostol pessary and high-dose vaginal misoprostol tablets (which was among the best treatments for efficacy) were most likely to increase the odds of excessive uterine activity..” p.105 |
| Intervention vs. Placebo | NMA OR | NMA 95% Crl |
|---|
| Double-balloon or Cook’s catheter | 0.26 | 0 to 1.18 |
| Laminaria | 0.52 | 0.01 to 2.62 |
| Foley catheter | 0.92 | 0.37 to 1.93 |
| Misoprostol oral less than 50 mcg | 1.13 | 0.28 to 3.15 |
| Dinoprostone vaginal NR pessary | 1.4 | 0.37 to 3.68 |
| Dinoprostone intracervical | 1.7 | 0.37 to 3.68 |
| Misoprostol oral, titrated low-dose | 1.93 | 0.73 to 4.19 |
| Dinoprostone vaginal tablet | 1.99 | 0.73 to 4.19 |
| Oxytocin IV | 2.12 | 0.97 to 4.1 |
| Dinoprostone vaginal gel | 2.33 | 0.97 to 4.1 |
| Misoprostol vaginal less than 50 mcg | 2.75 | 1.36 to 5.04 |
| Misoprostol oral 50 mcg or more | 2.85 | 1.41 to 5.2 |
| Dinoprostone vaginal SR pessary | 2.97 | 1.36 to 5.73 |
| Misoprostol buccal/ sublingual | 4.25 | 1.71 to 9.02 |
| Misoprostol vaginal 50 mcg or more | 4.4 | 2.22 to 7.94 |
| Misoprostol SR vaginal pessary | 5.58 | 1.58 to 14.57 |
| Oxytocin IV with amniotomy | 7.44 | 0.27 to 40.66 |
| ten Eikelder et al., 201613 |
|---|
| Foley catheter versus misoprostol (risk ratio, 95% confidence interval) | “Our meta-analysis demonstrates that Foley catheter gives less hyperstimulation compared to misoprostol (any dose, any administration route), and there were fewer vaginal instrumental deliveries with a Foley catheter. Furthermore, the Foley catheter resulted in fewer cesarean deliveries for nonreassuring FHR than misoprostol, with a comparable total cesarean deliveries rate. There were no differences in neonatal outcomes such as arterial umbilical cord pH of less than 7.05, Apgar score of less than 7 at 5 minutes, and NICU admission.” p.627 |
| Outcome | Foley Catheter Events/Total | Misoprostol Events/Total | RR, 95% CI |
|---|
| Safety outcomes |
|---|
| Hyperstimulation with FHR changes | 37/1786 | 71/1805 | 0.54 [0.37-0.79] |
| Meconium-stained liquor | 191/1694 | 221/1693 | 0.86 [0.72-1.03] |
| Cesarean for NR-FHR | 81/1635 | 112/1634 | 0.72 [0.55-0.95] |
| Vaginal instrumental delivery for NR-FHR | 58/1175 | 77/1187 | 0.77 [0.55-1.06] |
| Post-partum hemorrhage | 94/1158 | 89/1167 | 1.06 [0.81-1.4] |
| Arterial pH less than 7.05 | 16/921 | 22/924 | 0.73 [0.39-1.38] |
| Apgar less than 7 at 5 minutes | 28/1422 | 28/1453 | 1.03 [0.62-1.69] |
| NICU admission | 66/1673 | 66/1697 | 0.99 [0.71-1.38] |
| Neonatal mortality | 3/1021 | 1/1024 | 3.01 [0.31-28.88] |
| Effectiveness outcomes |
|---|
| Cesarean delivery rate | 539/2106 | 469/2128 | 1.16 [1-1.34] |
| Cesarean delivery for failure to progress 1st stage | 171/1545 | 124/1545 | 1.29 [0.88-1.9] |
| Vaginal instrumental delivery | 139/1389 | 190/1400 | 0.74 [0.6-0.91] |
CI = confidence interval; Crl = credible interval; NR-FHR = nonreassuring fetal heart rate; mcg = micrograms; NICU = neonatal intensive care unit; NMA = network meta-analysis; NR = normal release; OR = odds ratio; RR = risk ratio; SR = sustained release
Table 11Summary of Findings of Included Primary Clinical Studies
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Osoti et al., 201817 |
|---|
| Labour, maternal, and neonatal outcomes | “In this randomized trial, a combination of extra-amniotic Foley’s catheter and vaginal misoprostol compared to vaginal misoprostol alone for cervical ripening and induction of labor did not statistically significantly reduce the rates of failed induction but shortened the induction-to-delivery interval by 4 h, reduced the number of doses of misoprostol administered, and there was no difference in risk of adverse maternal and early perinatal outcomes.” p. 5 of 8 |
| Outcome | Foley + vag miso (n=90) n, % | Vag miso (n=90) n, % | RR 95% CI | P |
|---|
| Failed induction | 8 8.9% | 10 11.1% | 0.8 0.33-1.93 | 0.619 |
| Maternal complications |
|---|
| PPH | 3 3.3% | 4 4.4% | 0.75 0.17-3.26 | 0.707 |
| Uterine rupture | 0 | 1 1.1% | - | - |
| Neonatal complications |
|---|
| NICU admission | 15 16.7% | 17 18.9% | 0.86 0.37-1.98 | 0.697 |
| Five min Apgar less than 7 | 7 7.8% | 6 6.7% | 1.18 0.32-4.44 | 0.773 |
| “Women randomized to the combined extra-amniotic Foley’s catheter and misoprostol group had a significantly shorter duration of time between the onset of induction of labor and delivery (induction-delivery-time) compared to those in the misoprostol only group (Fig. 2). The Kaplan–Meier survival curve showed a statistically significantly steeper and different rate of decline in the extra-amniotic Foley’s catheter plus misoprostol group (log-rank test P < 0.001) compared to the misoprostol alone group.” p. 5 of 8 |
| Pimentel et al., 201818 |
|---|
| Outcomes between misoprostol dose treatment groups | “ shows that there were no statistically signifcant differences in the other secondary maternal and neonatal outcomes between the 2 groups. […] Our study demonstrated that there was no difference in the proportion of women who delivered vaginally within 24 hours with either a 1-misoprostol dose or multiple-misoprostol dose regimen. A Poisson regression analysis showed that treatment group assignment did not increase the cesarean delivery risk. Rather, the greatest risk factor for cesarean birth was a Bishop score <4 before the start of oxytocin. We found no difference between the 2 treatment groups in the Bishop score before the initiation of oxytocin. The use of a single dose of misoprostol for cervical ripening before the start of induction of labor with oxytocin, if clinically appropriate, is an acceptable alternative to the multiple-dose regimen.” p. 614.e6 |
| Outcome | 1 dose (n=120) | 2 doses (n=123) | P value |
|---|
| Vaginal delivery rates and timing |
|---|
| Vaginal delivery within 12 hours, n | 20 16.7% | 18 14.6% | 0.725 |
| Vaginal delivery within 24 hours, n | 50 41.7% | 55 (44.7% | 0.698 |
| Delivery within 24 hours, n | 61 50.8%) | 64 (52% | 0.898 |
| Time to vaginal delivery, median min | 1187 695-1628 | 1321 900.5-1735 | 0.202 |
| Time to delivery, median min | 1410 909.2-2070 | 1407 927-1958 | 0.775 |
| Cesarean delivery, n | 43 35.8% | 28 22.8% | 0.034 |
| CS, nulliparous, n | 35 49.3% | 20 28.6% | 0.016 |
| CS, multiparous | 8 16.3% | 8 15.1% | 1 |
| Oxytocin use |
|---|
| Oxytocin used, n | 103 85.8% | 95 77.2% | 0.099 |
| Bishop score before oxytocin, n | 4 (2-5) | 5 (3-6) | 0.124 |
| Maternal outcomes |
|---|
| Epidural dilation less than 4, n | 65 63.7% | 63 61.8% | 0.885 |
| Tachysystole, n | 7 5.9% | 8 6.6% | 1 |
| Chorioamnionitis, n | 20 16.7% | 15 12.2% | 0.364 |
| Endometritis, n | 6 5% | 4 3.3% | 0.536 |
| PPH, n | 19 16% | 14 11.4% | 0.351 |
| Blood transfusion, n | 6 5% | 5 4.1% | 0.767 |
| ICU admission | 1 0.8% | 0 | 0.494 |
| Uterine rupture | 0 | 1 0.8% | 0.972 |
| 3rd or 4th degree laceration | 2 2.6% | 5 5.2% | 0.226 |
| Neonatal outcomes |
|---|
| Apgar score at 5 min less than 6, n | 0 | 1 0.8% | 1 |
| pH less than 7.1, n | 2 1.8% | 6 5.2% | 0.281 |
| NICU admission | 13 10.8% | 7 5.7% | 0.166 |
| Sepsis | 0 | 1 0.8% | 1 |
| Pourali et al., 201819 |
|---|
| Labour outcomes | “The mean time between the start of labour induction or augmentation and the start of active labour was shorter in the misoprostol group than in the oxytocin group, but the difference was not statistically significant (p..439); the mean duration of active labour and the second stage of labour were significantly shorter in the misoprostol group (p=.036 and p=. 028, respectively)[…] Results from this study showed that the time between the induction of labour and the active labour was not significantly shortened by the use of sublingual misoprostol in comparison with oxytocin infusion (p=.299).[…] The frequency of uterine tachysystole and FHR abnormalities were significantly higher in the misoprostol group, as similar to some studies[…]” p. 169-71 |
| Outcome | Misoprostol Mean ± SD | Oxytocin Mean ± SD | P |
|---|
| Duration between start of IOL (or augmentation) until active labour (minutes) | N = 120 284.96 ± 197.51 | N = 120 229.54 ± 166.88 | 0.439 |
| Active labour duration in min (Median IQR) | N = 99 480 (330-705) | N = 97 600 (390-795) | 0.036 |
| Second stage of labour duration in min (Mean ± SD) | N = 99 48.43 ± 27.84 | N = 97 56.99 ± 26.46 | 0.028 |
| Conde et al., 201714 |
|---|
| Bishop score, neonatal outcomes | “There is no statistically significant difference in terms of the route of administration, sublingual or vaginal, for cervical ripening when using misoprostol 50 μg” p. 843 |
| Outcome | Sublingual misoprostol | Vaginal misoprostol | P |
|---|
| Bishop score at 6 hours -median (SD) | 5.47 (3.66) | 5.25 (3.46) | 0.761 |
| Apgar score at 1 minute -N | 8.39 1.3% | 8.63 0.6% | 0.242 |
| Apgar score at 5 minutes -N | 9.57 0.81% | 9.73 0.57% | 0.259 |
| pH cord blood gas -N | 7.23 0.85% | 7.23 0.95% | 0.927 |
| Maternal outcomes |
| Outcome | SL MPN | Vag MPN | RR | CI | P |
|---|
| Tachy-systole | 1 1.96% | 3 5.88% | 3.125 | 0.314-31.094 | 0.617 |
| CS | 10 21.28% | 7 13.73% | 1.533 | 0.534-4.405 | 0.635 |
| Further miso required | 41 80.39% | 37 72.55% | 0.645 | 0.256-1.626 | 0.872 |
| Labour within 6 h | 8 15.69% | 7 13.73% | 0.855 | 0.285-2.564 | 0.078 |
| Husain et al, 201715 |
|---|
Outcome parameters in the two interventions (P < 0.05 is significant) | “Our study shows that a combination of Foley’s balloon cathett and oral misoprostol reduces the failure rate of achieving vaginal delivery within 24 h of induction and decreases the induction-to-delivery interval. The number of doses of misoprostol required for induction is lower with a combination approach. NICU admissions and Apgar scores of <7 were also less common in our combination group. When stratifying according to parity, these differences remained significant only for the parous group," p. 1275 |
| Outcomes | Misoprostol alone (n=157) | Misoprostol + Foley (n=161) | P |
|---|
Delivery mode CS Vag delivery | 33 (21%) 124 (79%) | 13 (8.1%) 148 (91%) | 0.001 |
| Failure to deliver within 24h of IOL | 45 (28.7%) | 19 (11.8%) | 0.001 |
Indication for CS Non-reassuring FH pattern Failure to progress Failed induction | 15 (45.5%) 5 (15.2%) 13 (39.4%) | 6 (46.2%) 4 (30.8%) 3 (23.1%) | 0.391 |
| ITD interval median hours (range) | *19 (12-32) | *13 (11-29) | 0.001 |
Maternal complications Endometritis Hyperstimulation | 3 (1.9%) 11 (7%) | 1 (0.6%) 7 (4.3%) | 0.337 |
| NICU admissions | 26 (16.6%) | 11 (6.8%) | 0.007 |
| *These results were reversed in but were reported correctly in narrative. |
| Mundle et al., 201716 |
|---|
| Birth outcomes | “Our findings show that women undergoing labour induction with oral misoprostol had a quicker labour, were more likely to have a vaginal birth, and were more satisfied than those induced with the Foley catheter. Rates of uterine hyperstimulation were low in both groups and neonatal morbidity did not differ between groups.” p.675 |
| Outcome | Foley (n=300) | Miso (n=302) | AD (95% CI) | P |
|---|
| Vaginal birth within 24 hours | 141 (45%) | 172 (57%) | 10% (−2.0 to 17.9) | 0.0136 |
| CS | 151 (50.3%) | 124 (41.1%) | −9.2% (−17.2 to −1.3) | - |
| Uterine hyper-stimulation | 1 (0.3%) | 2 (0.7%) | 0.3% (−0.8 to 1.5) | 0.566 |
| FHR abnormality | 17 (5.7%) | 12 (4%) | −1.7% (−5.1 to 1.7) | 0.332 |
| Mean minutes (SD) from induction to birth – all women | 861 (468) | 771 (421) | -90 (-161 to-19) | 0.0134 |
| Rouzi et al., 201720 |
|---|
| Labour, maternal, and neonatal outcomes | “The use of oxytocin was similar between the hourly titrated-dose group (n. 54; 73.9%) and the static dose every 2 hours group (n. 52; 71.2%; P=.85). In addition, the proportions of women who were induced successfully after receiving oxytocin were similar between groups (P. .83). Frequencies of maternal adverse events were similar between groups, except for pyrexia[…] |
| Labour outcomes |
|---|
| Outcomes | Hourly miso (n = 73) | 2 hourly miso (n = 73) | P value | RR (95% CI) |
|---|
| Vag delivery in 24 h or less | 47 (64.4%) | 48 (65.8%) | 1.00 | 0.98 (0.77-1.24) |
| CS | 17 (23.2%) | 6 (8.2%) | 0.02 | 2.83 (1.18-6.77) |
| Oxytocin use | 39/54 (72.2%) | 39/52 (75%) | 0.83 | - |
| Maternal adverse events |
|---|
| Uterine tachysystole | 8 (10.9%) | 2 (2.7%) | 0.09 | - |
| Uterine hyperstimulation | 1 (1.37%) | 0 | 1.00 | - |
| Neonatal outcomes |
|---|
| Non-reassuring FHR | 16 (21.9%) | 10 (13.7%) | 0.28 | |
| Meconium-stained fluid | 23 (31.5%) | 23 (31.5%) | 0.05 | |
| Apgar <7 at 5 min | 1 (1.37%) | 0 | 1.00 | |
| NICU admission | 4 (5.48%) | 2 (2.74%) | 0.68 | |
| Wang et al., 201621 |
|---|
| Labour, maternal, and neonatal outcomes | “[…] titrated oral misoprostol given hourly for labor induction is as effective as vaginal dinoprostone from the respect of maternal/fetal outcomes, but titrated oral misoprostol is associated with lower rate of uterine hyperstimulation, hypertonus, tachysystole, and nonreassuring fetal heart compared with vaginal dinoprostone.” p. 503 |
| Outcome | Oral miso (n = 212) | Dinoprostone (n= 199) | P value |
|---|
| Vag delivery | 182 (85.8%) | 163 (81.9%) | 0.93 |
| Cesarean | 30 (14.2%) | 36 (18%) | 0.34 |
| Oxytocin use | 32/182 (17.6%) | 28/163 (17.1%) | 1.00 |
| Tachysystole | 15 (7%) | 19 (9.5%) | 0.61 |
| Hyper-stimulation | 5 (2.4%) | 18 (9%) | 0.03 |
| Uterine rupture | 0 | 0 | - |
| PPH | 14 (6.6%) | 15 (7.5%) | 0.73 |
| Non-reassuring FHR | 15 (7.1%) | 22 (11.1%) | 0.04 |
| Meconium-stained liquor | 46 (21.7%) | 55 (27.6%) | 0.58 |
| NICU admission | 15 (7%) | 15 (7.5%) | 1 |
| Yenuberi et al., 201622 |
|---|
| Labour Outcomes | “The main findings of this study are that the primary outcome, which was vaginal delivery within 24 h, was similar in both groups. Looking at secondary outcomes pertaining to effectiveness, women treated with stepwise oral misoprostol were more likely to labour without the use of oxytocin when compared to vaginal misoprostol. Women treated with stepwise oral misoprostol were also less likely to need a third dose when compared to women treated with vaginal misoprostol. The mean duration from IOL to delivery and mode of delivery were similar in both groups. Outcomes relating to complications in the fetus, neonate and the mother were similar in both groups,” p.202-3 |
| Outcomes | Vag miso n=380 | Oral miso n=383 | P | RR 95% CI | NNT |
|---|
| VD in 24 hours (n) | 254 67)% | 257 66.8% | 0.958 | 1.01 0.75-1.36 | 500 |
| Bishop score at ARM (median IQR) | 5 (5-7) | 5 (5-7) | 0.23 | - | - |
| Duration lOL to delivery (median IQR) | 18 h (13-22) | 17.53 (12-21) | 0.117 | - | - |
| VD within 12 h (n) | 53 13.9% | 67 17.5% | 0.102 | 0.76 0.55-1.05 | 28 |
| CS | 81 21.3% | 95 24.8% | 0.224 | 0.85 0.65-1.10 | 29 |
| Oxytocin use | 305 80.3% | 281 73.4% | 0.026 | 1.09 1.01-1.18 | 15 |
| Miso doses (median IQR) | 2 (2-3) | 2 (2-3) | 0.067 | - | - |
AD = absolute difference; ARM = artificial rupture of membranes; Cl = confidence interval; CS = cesarean section; FHR = fetal heart rate; h = hours; SMP = misoprostol; D = standard deviation; FH = fetal heart; FHR = fetal heart rate; IOL = induction of labour; ITD = Induction-to-delivery; IOL = induction of labour; IQR = interquartile range; min = minutes; miso = misoprostol; N = numbers; NNT = numbers needed to treat; SL = sublingual; P = probability; PPH = postpartum hemorrhage; RR = relative risk; SD = standard deviation; vag = vaginal; VD = vaginal delivery
Table 12Summary of Findings of Included Economic Evaluations
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Leigh et al., 201823 |
|---|
| “57% [95% CI (51.4-62.5%)] of our oral misoprostol group, as opposed to 47% [95% CI (41.5-52.8%)] in the Foley group, achieved a vaginal delivery within 24 hours of induction (P = 0.0162), while mean treatment costs equalled $138.10 per patient [95% CI %127.06-%146.28] in the Foley group, reducing by 14.9% to $117.51 per patient [95% CI %111.06-%123.45] in the oral misoprostol group.[…] Sensitivity analysis demonstrated a 63% probability of oral misoprostol being cost-saving over Foley catheterisation, and a 90.7%, 98.7%, and 99.4% probability of achieving superior rates of delivery within 24 hours of induction, vaginal delivery, and vaginal delivery within 24 hours of induction, respectively.”
p.6-7 | “The results of this multicentre randomised trial, performed in two hospitals within the Maharashtra Province of India, demonstrate that for the induction of hypertensive women in low-resource settings, low-dose oral misoprostol 25 meg is both more clinically effective and less resource-intensive than transcervical Foley catheterisation.[…] The results of this study suggest that when compared to Foley catheterisation for the induction of high-risk hypertensive women, oral misoprostol improves rates of vaginal delivery, delivery within 24 hours of induction, and vaginal delivery within 24 hours of induction and may also reduce costs.” p.6, 8 |
| ten Eikelder et al., 201826 |
|---|
| Costs per women (2013 €) | “Our analyses showed that the mean costs per woman were €4470 in the misoprostol group and €4158 in the Foley catheter group, a difference that was not statistically significant (mean difference €312, 95% CI €508 to €1063). Cost differences predominantly originated from duration of labour ward stay. The primary clinical outcome (composite risk of asphyxia and/or postpartum haemorrhage), as also caesarean section rate, were not significantly different. Therefore, when inducing labour with oral misoprostol instead of Foley catheter, there were no meaningful incremental costs to avoid one case of asphyxia, postpartum haemorrhage or caesarean section. Sensitivity analyses demonstrated that when estimates for resource use and unit prices are varied, the difference between both induction methods remained low.” p. 381 |
| Oral misoprostol (n = 924) | Foley catheter (n = 921) |
|---|
| Mean 2013 € | Median (IQR) | Mean 2013 € | Median (IQR) |
|---|
| Total delivery cost | 3047 | 2241 (1495-3450) | 2724 | 2173 (1476-3455) |
|---|
| Total postpartum cost | 1423 | - | 1434 | - |
|---|
| Total direct medical cost | 4470 | - | 4158 | - |
|---|
| Differential mean cost (95% CI) | - | - | - | 312 (-180 to 365) |
|---|
| Alfirevic et al., 201612 |
|---|
| Base case at a £20,000 willingness-to-pay threshold | “In summary, the base-case analysis found that all of the methods of induction were cost-saving compared with no treatment. It is noteworthy that there is considerable uncertainty in our cost-effectiveness estimates, with the majority of the interventions having very similar utility values, and mainly differing in total costs. With this caveat, buccal/sublingual misoprostol and titrated oral misoprostol were identified as being the interventions with the highest expected net benefit and the highest probability of being cost-effective. At any willingness-to-pay value of > £23,000 per unit increase in utility, titrated low-dose oral misoprostol solution seems to be the intervention that is most likely to be the most cost-effective for use on the UK NHS. Given that we were able to analyse only two subgroups (intact membranes and unfavourable cervix), and the number of interventions compared - and studies included - were lower than in the base case, the results of subgroup analyses should be interpreted cautiously (i.e. as hypothesis generating). In the subgroup of women with intact membranes, and limiting to interventions feasible on the NHS, i. v. oxytocin with amniotomy was identified as being the intervention with the highest expected net benefit and the optimal intervention at any willingness-to-pay value. However, there was again a lot of uncertainty in this estimate, with buccal/sublingual misoprostol and titrated (low-dose) oral misoprostol also with a moderate probability of being most cost-effective. Buccal/sublingual misoprostol and titrated low-dose oral misoprostol solution were found to be the interventions that were most likely to be cost-effective in women with an unfavourable cervix. The majority of the interventions, with a few notable exceptions, such as intracervical PGE2, result in similar expected utility and vary mainly in terms of cost. There is a considerable degree of uncertainty in these estimates, demonstrated by the wide confidence intervals around the values.” p. 102-3 |
| Treatment | Expected total cost £ (95% CI) | Expected total utility (95% CI) | Expected net benefit £ | ICER £ |
|---|
| Miso buccal/ SL | 1747.18 (1341.57 to 1472.34) | 0.821 (0.68 to 0.95) | 14,668.72 | Dominated |
| Oxytocin IV + amniotomy | 1747.80 (1275.41 to 2370.82) | 0.82 (0.67 to 0.95) | 14,652.13 | Dominated |
| Miso vag > 50 mcg | 1747.80 (1275.41 to 2370.82) | 0.82 (0.68 to 0.95) | 14,652.13 | Dominated |
| Miso oral titrated low-dose | 1747.80 (1275.41 to 2370.82) | 0.823 (0.68 to 0.96) | 14,658.28 | 21,190 |
| Miso vag < 50 mcg | 1747.80 (1275.41 to 2370.82) | 0.819 (0.68 to 0.95) | 14,533.98 | Dominated |
| Miso oral > 50 mcg | 1906.19 (1499.21 to 2384.89) | 0.819 (0.68 to 0.95) | 14,533.98 | Dominated |
| PGE2 vag gel | 1935.79 (1517.97 to 2429.53) | 0.817 (0.67 to 0.95) | 14,533.98 | Dominated |
| Foley | 1968.64 (1550.28 to 2463.38) | 0.815 (0.67 to 0.95) | 14,533.98 | Dominated |
| Oxytocin IV | 1977.39 (1536.48 to 2518.6) | 0.809 (0.66 to 0.95) | 14,533.98 | Dominated |
| MVISR | 1997.08 (1480.46 to 2597.86) | 0.805 (0.65 to 0.95) | 14,533.98 | Dominated |
| PGE2 vag insert NR | 2015.76 (1569.43 to 2533.94) | 0.811 (0.66 to 0.95) | 14,533.98 | Dominated |
| PGE2 intracervical | 2033.03 (1614.6 to 2532.76) | 0.633 (0.53 to 0.74) | 14,533.98 | Dominated |
| PGE2 vag SR pessary | 2036.15 (1602.91 to 2551.89) | 0.81 (0.66 to 0.95) | 14,533.98 | Dominated |
| PGE2 vag tab | 2042.64 (1638.01 to 2565.19) | 0.805 (0.65 to 0.95) | 14,533.98 | Dominated |
| Double-balloon or Cook’s catheter | 2097.74 (1618.43 to 2682.1) | 0.8 (0.64 to 0.95) | 14,533.98 | Dominated |
| Miso oral < 50 mcg | 2140.28 (1644.79 to 2738.28) | 0.802 (0.64 to 0.94) | 14,533.98 | Dominated |
| Bierut et al., 201624 |
|---|
MVI compared with standard of care Negative values are cost savings. | “The results showed that IOL [induction of labour] with MVI generates savings, from a hospital perspective, in the majority of comparisons performed with other prostaglandins. Unsurprisingly, the difference in favor of MVI originated in the longer time of the induction phase and the labor itself for other prostaglandins. A shorter stay of the patient in the ward is directly connected with a shortening of the time of medical staff care.” p. 1766 |
| Country | Comparator with MVI | Cost-difference / patient (€) | Statistically significant cost-difference/ patient (€) |
|---|
| Austria | MVI vs DVI MVI vs PGE2 tab | −713.42 −575.13 | −684.80 −649.24 |
| Poland | MVI vs PGE2 gel MVI vs Foley | −302.38 24.30 | −151.00 87.62 |
| Russia | MVI vs PGE2 gel | −23.04 | 5.36 |
| Slovakia | MVI vs PGE2 tab MVI vs PGE2 gel | −198.35 −425.12 | −233.39 −387.67 |
| Draycott et al., 201625 |
|---|
| Model parameters as per EXPEDITE trial (published and unpublished data) | “Our model, based on data from real-life clinical practice and a large clinical trial, demonstrates that the MVI, by decreasing time to delivery compared with the DVI, may have a positive impact on hospital resource use and capacity. We estimate that using the MVI, rather than the current recommended standard of care, could reduce the number of midwife shifts required for labour induction and delivery, as well as the level of labour and delivery suite occupancy necessary to manage labour-induced births. This is due to the reduced time to active labour and vaginal delivery associated with the use of the MVI compared with the DVI, which in turn decreases the overall need for vital signs monitoring and vaginal examinations-in particular due to a reduction in the rate of, and duration of, oxytocin use (the administration of which requires continuous monitoring to check the progress of cervical ripening 12,30]). p. 49 |
| Model parameter | DVI (n=680) | MVI (n=678) |
|---|
| Time in hours to vaginal delivery - all patients | 36,653 | 25,461 |
| Birth within 12 hours of induction | Parous: 18.3% Nulliparous: 1.6% | Parous: 35.6% Nulliparous: 5.9% |
| Birth within 24 hours of induction | Parous: 54.3% Nulliparous: 13.1% | Parous: 70% Nulliparous: 27.1% |
| Number of midwife shifts over 1 year | Parous: 81 Nulliparous: 124 | Parous: 59 Nulliparous: 94 |
| Hours in L&D suite | Parous: 14,581 Nulliparous: 24,938 | Parous: 10,395 Nulliparous: 18,308 |
CI = confidence interval; DVI = dinoprostone vaginal insert; ICER = incremental cost-effectiveness ratio; L&D = labour and delivery; miso = misoprostol; MVI = misoprostol vaginal insert; N = numbers; NR = normal release; PGE2 = dinoprostone; SL = sublingual; SR = sustained release; vag = vaginal; VD = vaginal delivery
Table 13Summary of Recommendations in Included Guidelines
View in own window
| Recommendations | Strength of Evidence and Recommendations |
|---|
| SOGC, 20131 |
|---|
| Misoprostol can be considered a safe and effective agent for labour induction for inpatients with intact membranes. | Quality of evidence: I (high) Evidence obtained from at least one properly randomized controlled trial. Recommendation class: A (strong) There is good evidence to recommend the clinical preventive action. |
| Misoprostol should not be used for vaginal birth in patients with previous Caesarean section due to the increased risk of uterine rupture. | Quality of evidence: II-3 (low) Evidence obtained from comparisons between times or places with or without the intervention. Dramatic results in uncontrolled experiments could also be included in this category Recommendation class: D (moderate) There is good evidence to recommend the clinical preventive action. |
| Oxytocin should be started no earlier than 4 hours after the last dose of misoprostol. | Quality of evidence: III (low) Expert opinion. Recommendation class: B (weak) There is fair evidence to recommend the clinical preventive action. |
Appendix 5. Overlap between Included Systematic Reviews
Table 14Primary Study Overlap between Included Systematic Reviews
View in own window
| Primary Study Citation | Systematic Review Citation |
|---|
| *Alfirevic et al., 2016 Year12 | *Ten Eikelder et al., 201613 |
|---|
| Barrilleaux 2002 | | X |
| Carbone 2013 | | X |
| Chavakula 2015 | | X |
| Chung 2003 | X | X |
| Eikelder 2015 | | X |
| Filho 2010 | X | X |
| Gelisen 2004 | X | X |
| Greybush 2001 | X | X |
| Hill 2009 | | X |
| Jozwiak 2014 | X | X |
| Kandil 2012 | X | X |
| Kashanian 2006 | X | X |
| Lanka 2014 | | X |
| Noor 2015 | | X |
| Oliveira 2010 | X | X |
| Owolabi 2005 | X | X |
| Prager 2008 | X | X |
| Roudsari 2011 | X | X |
| Rust 2001 | | X |
| Sciscione 2001 | X | X |
| Sheiker 2009 | X | X |
| Sujata 2012 | | X |
- *
Only those Alfirevic studies which show overlap with the ten Eikelder meta-analysis are included. All ten Eikelder studies are listed.
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Misoprostol for Cervical Ripening and Induction of Labour: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines. Ottawa: CADTH; 2018 Nov. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.