NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US). Evidence-Based Medicine and the Changing Nature of Healthcare: 2007 IOM Annual Meeting Summary. Washington (DC): National Academies Press (US); 2008.

Evidence-Based Medicine and the Changing Nature of Healthcare: 2007 IOM Annual Meeting Summary.
Show detailsINTRODUCTION
Many Americans assume that the health care they receive is based on strong medical evidence of intervention and treatment effectiveness. However, as suggested by regional analyses, recommended care is often not delivered and insufficient evidence often leads to wide practice variations with little to no health benefit to patients (Fisher et al., 2003b; McGlynn et al., 2003). In addition to negatively impacting health outcomes, practice inconsistencies have dramatic effects on the overall costs of health care—costs which represent the most pressing fiscal challenge to the nation. Papers in this chapter examine the drivers of practice variations and healthcare costs and suggest the potential for an improved evidence base to improve the efficiency and effectiveness of healthcare services.
The majority of U.S. healthcare expenditures today are related to the care and treatment of chronic conditions such as heart disease, diabetes, and asthma, which affect almost half of the U.S. population. Evidence for effective strategies for care delivery in these areas is limited, resulting in care that is fragmented, uncoordinated, and characterized by unnecessary duplication of services. In Elliott S. Fisher’s paper, small-area analyses reveal that differences in care delivery explain almost all of the geographic variations in spending across the United States, and that higher-spending regions of the country perform worse in measures of technical quality than regions that spend less money. When there is strong medical evidence, physicians tend to agree on courses of treatment across regions of different spending levels. Building an evidence base for areas in which physicians currently use their own discretion, such as the comparative effectiveness of various treatment options, or decisions about how often to see a patient with well-controlled hypertension and when to order certain medical tests, could greatly improve the quality of care and reduce costs. In fact, if all regions adopted the practice patterns of the most conservatively spending regions of the country, health outcomes could be significantly improved and U.S. healthcare spending could decline by as much as 30 percent.
Peter R. Orszag’s presentation illustrates the serious consequences that excessive healthcare spending poses to the nation’s economic well-being. If spending growth trends continue as they have over the past four decades, by 2050 Medicare and Medicaid spending will account for 20 percent of the total U.S. economy. Although often ascribed to effects of an aging population, lower fertility rates, and longer life expectancies, this long-term fiscal challenge is driven almost entirely by excessive healthcare costs—or costs per beneficiary. Slowing overall healthcare cost growth without limiting access will require changes that impact the overall healthcare system and providing better evidence to inform decision making will be an important first step. Comparative effectiveness research that draws upon the emerging electronic health record and clinical registry data resources may be the only cost-effective and feasible mechanism for bringing about the evidence-base expansion needed. Real gains in improving the quality of health care and reducing costs will come when the evidence of medical effectiveness is tied to incentive payments for healthcare providers. A combination of increased cost sharing on the consumer side combined with changes in the incentive system for providers informed by best evidence offers an important opportunity to substantially reduce healthcare costs and improve quality.
HEALTH CARE AND THE EVIDENCE BASE
Elliott S. Fisher, Dartmouth Medical School
The U.S. healthcare system faces serious challenges and the Institute of Medicine (IOM) has played a critical role in calling for fundamental transformation of the delivery system to achieve the vision of a patient-centered, high-quality, equitable, and effective delivery system (IOM, 2001, 2006). There is also a growing recognition that our current delivery system is failing to deliver on the promise of improved health offered by advances in biomedical knowledge, and the future pace of change may widen this gap substantially.
The IOM Roundtable on Evidence-Based Medicine was established “to help transform the way evidence on clinical effectiveness is generated and used to improve health and health care” (IOM, 2007a). The key notions are to provide better evidence about the risks and benefits of interventions and to support better application of that knowledge to clinical practice. Several recent reports highlight the growing consensus on the need for expanded support for comparative effectiveness research that provides better information about the risks and benefits of specific treatments (IOM, 2007b).
This paper draws on the traditions of small-area analysis to underscore the scope of the challenge faced in bringing evidence to bear on current practice and to point to the opportunity for improving both the costs and the quality of care by ensuring a broad definition of the need for evidence.
Categories of Care: Biologically Targeted Interventions Versus Care Management Strategies
As we consider the relationship between evidence and clinical practice, it is worth considering two broad categories of interventions: discrete, biologically targeted interventions and care delivery strategies.
Biologically targeted interventions are focused on a specific anatomic problem or disease process. Examples include the decision about whether to adopt a specific screening test for cancer or whether to treat a patient with prostate cancer with surgery or radiation therapy. Such interventions can be well specified not only in terms of the underlying anatomic or physiologic problem to be addressed, but also in terms of the expected intermediate and long-term outcomes and how these vary across clinical subgroups. Many of the dramatic improvements in health outcomes achieved over the past decades are a result of the advances in biomedical knowledge and the development of such biologically targeted interventions. These are the traditional focus of technology assessment, clinical guidelines, and a narrow definition of “evidence-based practice.”
A second category of “decisions,” which I refer to as care delivery strategies, is rarely considered explicitly in the day-to-day practice of clinical medicine. This category refers not to what care is provided (what drug, what device, what surgical procedure) or to whom (which patients should be offered the intervention), but to how a specific biologically targeted therapy is delivered: who should provide the care (patients themselves, advanced practice nurses, primary care physicians, or specialists); where care should be delivered (home, outpatient facility, or hospital); and how intensively patients should be monitored and reevaluated. Questions about care delivery also encompass system- and policy-level issues, such as how care should be organized, what kinds of resources should be deployed, how care should be paid for and financed, and how to improve the quality of care.
There are three reasons to distinguish these two categories of decisions. First, the effectiveness of many (if not most) discrete biologically targeted interventions can depend critically on how care is provided: risk-adjusted surgical mortality rates, for example, vary severalfold across hospitals (Birkmeyer et al., 2002; O’Connor et al., 1991). Second, as discussed below, regional and provider-specific differences in costs are largely due to differences in the intensity of care delivery: addressing the rapidly rising costs of care will require much better evidence about how to organize and deliver care effectively and efficiently. Finally, I argue that the infrastructure required to provide better evidence on discrete, biologically targeted interventions is fundamentally the same as the infrastructure required to improve care delivery. As we build our capacity to improve evidence, we should be careful to address the need for better evidence along both dimensions.
Current Practice and the Evidence Base: Biologically Targeted Interventions
The recent IOM workshop on evidence-based medicine highlighted the many limitations of the current evidence base, focusing primarily on the challenges surrounding biologically targeted therapies (IOM, 2007a). Highlights include the lack of any evidence on the efficacy or effectiveness of many interventions, the difficulty of extrapolating from trials carried out on selected populations to those with multiple chronic conditions, and the growing recognition that the benefits of interventions vary according to the underlying risk of the population: trials that show benefit for the average patient may not reveal that many lower-risk patients may be harmed by receiving the procedure while those at greater risk receive substantial benefits. The growing recognition of the importance of comparative effectiveness research can be attributed to the increasing attention focused on these issues.
The relative magnitude of the uncertainty surrounding the use of selected, discrete, biologically targeted therapies can be illustrated by the regional variations in rates of these services among the Medicare population (Figure 2-1). We have found it useful to distinguish effective care (treatments where the evidence of benefit is strong and no trade-offs among benefits and harms are involved) from preference-sensitive care (treatments where patients’ values about the different outcomes may vary1) (Wennberg et al., 2002). An example of the former would be hospitalizations for hip fracture: the diagnosis is straightforward and the therapy (inpatient surgical repair of the fracture) is required. Variations in utilization rates are due entirely to underlying variations in the incidence of the disease. Examples of the latter would include screening for prostate cancer (where patient attitudes toward the risks of treatment must be weighed against the still unproven benefits of screening) or percutaneous coronary interventions for stable angina (where the modest benefit in terms of angina relief must be weighed against the lifelong need for anti-platelet therapy among other risks). When we look at common biologically targeted interventions—both diagnostic and therapeutic—we see dramatic variability across the United States. Addressing these variations will require not only better information about risks and benefits (comparative effectiveness research), but also ensuring that treatment decisions reflect the well-informed judgments of patients rather than the opinions of providers (O’Connor et al., 2007; Wennberg et al., 2007).
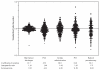
FIGURE 2-1
Variation in utilization rates of specific, biologically targeted interventions. NOTE: Each dot represents the ratio of the rate of the specified intervention in one of the 306 U.S. Hospital Referral (more...)
Current Practice and the Evidence Base: Care Delivery Strategies
There are also marked differences across regions in the way care is delivered (Figure 2-2). Although virtually all Medicare beneficiaries have access to care (defined as at least one physician visit during the year) and there is thus little regional variation in the age-, sex-, or race-adjusted rate of at least one physician visit, we see marked variability in the use of other care delivery strategies. Because variation in use of these services is associated with the local capacity of the delivery system (how many physicians, how many hospital beds), we have long referred to these services as “supply-sensitive.”
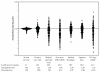
FIGURE 2-2
Variation in utilization rates of care delivery strategies. NOTE: Each dot represents the ratio of the rate of the specified service or strategy in one of the 306 U.S. Hospital Referral Regions to the U.S. (more...)
One of the fundamental reasons for distinguishing care delivery strategies from the use of biologically targeted interventions is their distinct relationship to variations in spending. Figure 2-3 displays the relationship between spending and the utilization rates of specific types of services across U.S. regions. Each dot represents the ratio of the utilization rates of the specific service in regions that fall in the highest quintile to the utilization rate in the lowest-spending quintile of regional per capita Medicare spending. To control for potential differences in the underlying health status of populations across regions, these analyses are based on long-term follow-up of patients initially hospitalized with hip fracture, colon cancer, or acute myocardial infarction (Fisher et al., 2003a). Higher spending is not associated with greater use of biologically targeted interventions: whether these are treatments that all patients should receive (effective care) or interventions where patients’ judgments about how they value the risks and benefits should determine the treatment choice (preference-sensitive care). Higher spending, however, is largely due to differences in care delivery: how frequently patients are seen (evaluation and management services), how much time they spend in the hospital, and the intensity with which they are monitored (diagnostic tests and imaging).

FIGURE 2-3
Ratio of utilization rates for selected specific services among cohorts of Medicare beneficiaries in high- versus low-spending regions. NOTE: High- and low-spending regions were defined as the U.S. Hospital (more...)
Spending, the Intensity of Care Delivery, and Health Outcomes
The critical question underlying the variations in practice and spending is their relationship to health outcomes. Over the past 10 years, a number of studies have explored the relationship between higher spending and the quality and outcomes of care (Table 2-1).
TABLE 2-1
Relationship Between Regional Differences in Spending and the Content, Quality, and Outcomes of Care.
Patients’ Experiences and Outcomes
Whether the study was carried out at the state level (Baicker and Chandra, 2004), across hospital referral regions (Fisher et al., 2003a), or across the major academic medical centers within the United States (Fisher et al., 2004), a consistent pattern is found: the quality of care as reflected in process measures of care is worse when spending—and the intensity of care delivery—is greater. Among patients hospitalized with hip fractures, colon cancer, and acute myocardial infarction who were followed for up to five years, mortality rates in higher-spending regions and hospitals were no better or slightly worse than in lower-spending delivery systems (Fisher et al., 2003a). In regions where spending growth was greatest, survival following myocardial infarction improved more slowly than in regions where spending growth was slower (Skinner et al., 2006). Finally, Medicare beneficiaries’ overall satisfaction with care was no better in higher-spending regions and their perceptions of the accessibility of care were somewhat worse (Fisher et al., 2003a).
Physician Attributes, Practice Settings, and Perceptions of Care
On a per capita basis, the highest-spending quintile of hospital referral regions have 65 percent more medical specialists per capita, 75 percent more general internists, and 25 percent fewer family practitioners than the lowest-spending quintile. A substantially higher proportion of physicians are foreign medical graduates, fewer are board certified, and they are much more likely to practice in small groups than physicians in lower-spending regions (Sirovich et al., 2006). When surveyed, physicians in higher-spending regions are more likely to report that the continuity of care with their patients is inadequate to support high-quality care and that the quality of communication is insufficient to support high-quality care. In spite of the substantially greater per capita supply of both beds and specialists, physicians in higher-spending regions are more likely to perceive scarcity: they are more likely to report that it is difficult to get a patient into the hospital and that it is hard to obtain adequate medical specialist referrals.
These findings are consistent with the hypothesis that the lower-spending regions represent a reasonable benchmark of efficiency. In fact, if all U.S. regions could safely adopt the organizational structures and practice patterns of the lowest-spending regions of the United States, Medicare spending would decline by about 30 percent (Fisher et al., 2003a; Wennberg et al., 2002). While it may not be realistic to reduce spending by that amount, the magnitude of the differences in practice and the fact that the differences in spending are largely due to differences in care delivery point to an important opportunity: improving efficiency will require attention not only to the comparative effectiveness of biologically targeted interventions, but also to addressing the underlying causes of the differences in care delivery across regions and systems.
Underlying Causes of the Differences in Care Delivery: Evidence and Theory
The Evidence
A number of studies have explored the underlying causes of the regional differences in spending and the intensity of care delivery. Patients’ preferences for care vary slightly across regions, but not enough to explain the magnitude of spending differences seen. For example, Medicare beneficiaries in high-spending regions are no more likely to prefer aggressive end-of-life care than those in low-spending regions (Barnato et al., 2007; Pritchard et al., 1998). Differences in the malpractice environment are associated with differences in both practice and spending, but explain less than 10 percent of state-level differences in spending and have a comparably small impact on differences in the growth in spending across states (Baicker et al., 2007; Kessler and McClellan, 1996). The role of capacity is clearly important, but the hospital bed supply and physician supply combined explain less than 50 percent of the difference in spending across regions (Fisher et al., 2004).
The most recent studies have focused on the use of clinical vignettes to explore how physicians’ judgments vary across regions of differing spending levels. These studies have found that physicians in higher-spending regions were no more likely to intervene in cases where evidence was strong (such as chest pain with an abnormal stress test), but were much more likely to recommend discretionary treatments (such as more frequent visits, referral to a specialist, or use of imaging services) than those in low-spending regions (Sirovich et al., 2005).
A Likely Diagnosis: Capacity, Payment, and Clinical Culture
These findings suggest a likely explanation for the dramatic differences in spending across regions and the paradoxical finding that higher spending seems to lead to worse quality and worse outcomes. Current clinical evidence is an important, but limited, influence on clinical decision making. Most physicians practice within a local organizational context and policy environment that profoundly influences their decision making, especially in discretionary clinical settings. Hospitals and physicians each face incentives that will in general reward expansion of capacity (especially for highly reimbursed services) and recruitment of additional procedure-oriented specialists. When there are more physicians relative to the size of the population they serve, physicians will see their patients more frequently. When there are more specialists or hospital beds available, primary care physicians and others will learn to rely upon those specialists and use those beds. (It is more efficient from the primary care physician’s perspective to refer a difficult problem to a specialist or to admit a patient to the hospital than to try to manage the patient in the context of an office visit for which payments have become relatively constrained.)
The consequence is that what—given the state of current evidence—are “reasonable” individual clinical and policy decisions lead in aggregate to higher utilization rates, greater costs, and inadvertently, worse quality and worse outcomes. The key element of this theory is that because so many clinical decisions are in the “gray areas” (how often to see a patient, when to refer to a specialist, when to admit to the hospital), any expansion of capacity will result in a subtle shift in clinical judgment toward greater intensity.
Implications for Evidence Development
These findings and their likely explanation point to the need for much better evidence. We need evidence about the risks and benefits of discrete, biologically targeted interventions and how these risks and benefits vary across different subgroups of the population, especially those often excluded from current randomized trials (IOM, 2007b), but we also need much better evidence about care delivery. No matter how good our clinical evidence about specific interventions becomes, many—if not most—clinical decisions will still require judgment. Also, because there will always be gray areas, we will need evidence that can guide clinicians, administrators, and policy makers when they are making decisions about care delivery.
Although the need for evidence may appear overwhelming, an important opportunity lies in recognizing that the information systems and analytic approaches required to improve the evidence base for biologically targeted interventions and for improving care delivery are fundamentally the same (Table 2-2). In the ideal world of improved information systems and electronic records that might allow relatively routine assessment of both short- and long-term health outcomes and effective follow-up of patients, the capacity to evaluate both care delivery and biologically targeted interventions would be critical, at least in part because lack of information about the local context (delivery system attributes) would sharply limit our ability to properly interpret studies of biologically targeted interventions.
TABLE 2-2
Relationship Between the Information and Approaches Required to Improve the Evidence Base Around Biologically Targeted Interventions and Care Delivery.
Moving Forward: A Challenge to Academic Medicine
The critical importance of healthcare spending to the future financial health of the U.S. government and the economy in general has received growing attention (Orszag and Ellis, 2007). Our capacity to provide affordable healthcare coverage to the U.S. population and our ability to pay for the new biologically targeted interventions that are under development will clearly depend not only on the costs of the interventions but also on the costs of delivering those interventions. Academic medicine—and the federal agencies that provide research support—have largely focused on improving our understanding of disease biology, while ignoring the need to understand and address the dramatic variations in care delivery among academic medical centers (Wennberg et al., 1987).
Table 2-3 points to the magnitude of the opportunity—and the challenge—for academic medicine. The upper portion of the table focuses on the degree to which each of these five members of the U.S. News and World Report’s “Honor Roll” of academic medical centers is able to deliver proven clinical interventions to eligible patients during an acute inpatient stay. The lower portion of the table highlights the differences in spending and overall intensity of care. The specific data focus on care provided in the last six months of life, but these patterns of practice are highly predictive of how these institutions treat other seriously ill patients. All five provide high-quality inpatient care. The differences in care delivery, however, are substantial: patients at the University of California, Los Angeles, have twice as many visits, spend about 50 percent more time in the hospital, and cost about twice as much as those treated at the Mayo Clinic in Rochester, Minnesota, or the Cleveland Clinic.
TABLE 2-3
Performance of Selected Major Academic Medical Centers on Measures of Adherence to Biologically Targeted Treatments and the Intensity of Care Delivery.
If all U.S. delivery systems could achieve the apparent efficiency of a Mayo or a Cleveland Clinic, the resources available to expand coverage to the uninsured or to provide interventions of proven benefit to those who are not be able to afford them would be substantial. Failure to address this challenge would call into question not only the scientific integrity of the enterprise (Are we really committed to asking important questions?), but also our moral authority as healthcare providers (How can we continue to ignore obvious opportunities to improve quality and the future affordability of care?).
Academic medicine has the opportunity to lead the development of a learning healthcare system. Such an effort should include a focus not only on the science of disease biology and improving the evidence to support the use of biologically targeted interventions, but also on the sciences of clinical practice and the evidence to support improvements in care delivery (Wennberg et al., 2007).
THE HIGH PRICE OF THE LACK OF EVIDENCE
Peter R. Orszag, Congressional Budget Office
The nation’s long-term fiscal challenge has largely been misdiagnosed in popular descriptions. It typically is described as being driven mostly by the aging of baby boomers, with lower fertility rates and longer life expectancy causing most of the long-term budget problem. In fact, most of that long-term problem is driven by excess healthcare cost growth—that is, the rate at which healthcare costs grow compared to income per capita. In other words, it is the rising cost per beneficiary, rather than the number of beneficiaries, that explains the bulk of the nation’s long-term fiscal problem.
You can see this phenomenon arising even over the next decade: Figure 2-4 shows the Congressional Budget Office’s (CBO’s) projections for spending on Social Security, Medicare, and Medicaid through 2017. As the figure shows, Social Security rises by about 0.5 percentage points of gross domestic product (GDP), from 4.2 percent of GDP to 4.8 percent over that period. Medicare and the federal share of Medicaid rises from 4.6 percent of GDP to 5.9 percent of GDP—an increase of 1.3 percentage points of GDP, or roughly twice as much as Social Security even over the next decade.
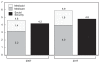
FIGURE 2-4
Spending on Medicare and Medicaid and on Social Security as a percentage of GDP, 2007 and 2017.
If you look over longer periods of time, the basic point is accentuated. Figure 2-5 shows a simple extrapolation in which Medicare and Medicaid costs continue to grow at the same rate over the next four decades as they did over the past four. (Even with no change in federal policy, there are reasons to believe that this simple extrapolation may overstate future cost growth in Medicare and Medicaid. CBO has recently released a long-term health outlook that presents a more sophisticated approach to projecting Medicare and Medicaid costs under current law, but a straight historical extrapolation is shown here for simplicity.) Under that scenario, Medicare and Medicaid would rise from 4.6 percent of the economy today to 20 percent of the economy by 2050; 20 percent of GDP is the entire size of the federal government today.
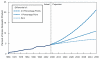
FIGURE 2-5
Total federal spending for Medicare and Medicaid under assumptions about the health cost growth differential. SOURCE: Congressional Budget Office, 2007.
The most interesting part of Figure 2-5 is the bottom line, which isolates the pure effect of demographics on those two programs. The only reason that the bottom line is rising is that the population is getting older and there are more beneficiaries on the two public programs. The increase between today and 2050 in that bottom dotted line shows that aging does indeed affect the federal government’s fiscal position. Yet that increase is much smaller than the difference in 2050 between the bottom line and the top line. In other words, the rate at which healthcare costs grow—whether they continue to grow at 2.5 percentage points per year faster than per capita income, or 1 percentage point, or 0.5 percentage point, is to a first approximation the central long-time fiscal challenge facing the United States.
It is common to say that the sooner we act the better off we are, and just to calibrate that, Figure 2-6 shows that if we slowed healthcare costs growth from 2.5 percentage points to 1 percentage point starting in 2015—which would be extremely difficult if not impossible to do, but is helpful as an illustration—the result in 2050 would be a reduction of 10 percent GDP in Medicare and Medicaid expenditures for the federal government relative to no slowing in the cost growth rate. That 10 percent of GDP difference is half of what the federal government spends today.

FIGURE 2-6
Effects of slowing the growth of spending for Medicare and Medicaid. SOURCE: Congressional Budget Office, 2007.
All of this may seem pretty challenging and is further complicated by the fact that it is implausible that we will slow Medicare and Medicaid growth in a sustainable way unless overall healthcare spending also slows. The reason is that if all you did was, say, to reduce payment rates under Medicare and Medicaid, and then try to perpetuate that over time without a slowing of overall healthcare cost growth, the result would likely be substantial access problems that would be inconsistent with the underlying premise and public understanding of these programs. One therefore needs to think about changes to Medicare and Medicaid in terms of the impact they can have on the overall healthcare system.
From that perspective, there appears to be a very substantial opportunity embedded in this long-term fiscal challenge facing the United States: the possibility of taking costs out of the system without harming health. Perhaps the most compelling evidence underscoring this opportunity is the significant variations across different parts of the United States that do not translate into differences in health quality or health outcomes (Figure 2-7).
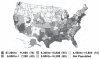
FIGURE 2-7
Medicare spending per capita in the United States, by hospital referral region, 2003. NOTE: The numbers in parentheses indicate the number of regions in each group. SOURCE: The Dartmouth Atlas Project, (more...)
The question then becomes, why is this happening? To me, it appears to be a combination of two things. One is the lack of information specifically about what works and what does not. The second thing is a payment system, on both the provider and the consumer sides, that accommodates the delivery of low-value or negative-value care.
On the consumer side, despite media portrayals to the contrary, the share of healthcare expenditures paid out of pocket—which is basically the relevant factor for evaluating the degree to which consumers are faced with cost sharing—has plummeted over the past few decades, from about 33 percent in 1975 to 15 percent today (Figure 2-8). All available evidence suggests that lower cost sharing increases healthcare spending overall, and collectively we all pay a higher burden, although the evidence is somewhat mixed on the precise magnitude of the effect by which lower cost sharing raises overall spending.
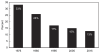
FIGURE 2-8
Share of personal healthcare expenditures paid out of pocket.
This observation leads some analysts to argue that the way forward is more cost sharing and a health savings account approach, and this can indeed help to reduce costs. However, two things need to be kept in mind in evaluating this approach. The first is that a significant amount of cost sharing is involved in existing plans. Moving to universal health savings accounts would thus not entail as great an increase in cost sharing, and therefore as much a reduction in spending, as you might think. Second, there is an inherent limit to what we should expect from increased consumer cost sharing because healthcare costs are so concentrated among the very sick. For example, the top 25 percent most expensive Medicare beneficiaries account for 85 percent of total costs, and the basic fact that healthcare costs are very concentrated among a small share of the population is replicated in Medicaid and in the private healthcare system. To the extent that we in the United States want to provide insurance, and insurance is supposed to provide coverage against catastrophic costs, the fact that those catastrophic costs are accounting for such a large share of overall costs imposes an inherent limit to the traction that one can obtain from increased consumer cost sharing. In sum, increased cost sharing on the consumer side can help to reduce costs, but it seems very unlikely to capture the full potential to reduce costs without impairing health quality.
This leads us to the provider side. On the provider side, the accumulation of additional information and changes in incentives could improve efficiency in the delivery of health care. There is growing interest in comparative effectiveness research, and the original House version of the State Children’s Health Insurance Program legislation had some additional funding for comparative effectiveness research. Other policy makers seem interested in expanding comparative effectiveness research. We need, though, to ask some hard questions about what we mean by comparative effectiveness research and how it would be implemented.
The key issues are what kind of research is undertaken and the standard of evidence used. As Mark McClellan has noted, comparative effectiveness research will very likely have to rely on nonrandomized evidence. The reason is that it seems implausible that we could build out the evidence base across a whole variety of different clinical interventions and practice norms using only randomized control trials, especially if we want to study subpopulations. On the other hand, economists have long been aware of the limitations of panel data econometrics, where one attempts to control for every possible factor that could influence the results—typically, that attempt is far from perfectly successful. There is thus a tension between using statistical techniques on panel data sets (of electronic health records, insurance claims, and other medical data), which seems to be the only cost-effective and feasible mechanism for significantly expanding the evidence base, and the inherent difficulty of separating correlation and causation in such an approach.
In terms of the budgetary effects of comparative effectiveness research, a lot depends on both what is done and how it is implemented. If the effort only involves releasing the results of literature surveys, the effects would likely be relatively modest. If new research using registries or analysis of electronic health records is involved, there may be somewhat larger effects. The real traction, though, will come from building the results of that research into financial incentives for providers. In other words, if we move from a “fee-for-service” system to a “fee-for-value” one, where higher-value care is awarded stronger financial incentives and low-value or negative-value health care is penalized by smaller incentives, or perhaps even penalties, the effects would be maximized. The design of such a system is very complicated and difficult to implement, but this is where the greatest long-term budgetary savings could come.
In conclusion, it is plausible to me that the combination of some increased cost sharing on the consumer side and a substantially expanded comparative effectiveness effort, combined with changes in the incentive system for providers, offers the nation the most auspicious approach to capturing the apparent opportunity to reduce healthcare costs at minimal or no adverse consequences for health outcomes. The focus of this publication is thus central to addressing the nation’s long-term fiscal challenge.
REFERENCES
- Baicker K, Chandra A. Medicare spending, the physician workforce, and beneficiaries’ quality of care. Health Affairs (Millwood). 2004:W4-184–4-197. Suppl Web Exclusives. [PubMed: 15451981]
- Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the Medicare program. Health Affairs (Millwood). 2007;26(3):841–852. [PMC free article: PMC2266679] [PubMed: 17485765]
- Barnato AE, Herndon MB, Anthony DL, Gallagher PM, Skinner JS, Bynum JP, Fisher ES. Are regional variations in end-of-life care intensity explained by patient preferences?: A study of the U.S. Medicare population. Medical Care. 2007;45(5):374–376. [PMC free article: PMC2147061] [PubMed: 17446824]
- Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. New England Journal of Medicine. 2002;346(15):1128–1137. [PubMed: 11948273]
- Congressional Budget Office. Research on the comparative effectiveness of medical treatments: Options for an expanded federal role. Testimony by Director Peter R. Orszag before House Ways and Means Subcommittee on Health. 2007. [accessed June 26, 2008]. http://www
.cbo.gov/ftpdocs /82xx/doc8209/Comparative _Testimony.pdf . - The Dartmouth Atlas Project. Medicare spending per capita in the United States, by hospital referral region. Dartmouth College; 2003. (unpublished).
- Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: The content, quality, and accessibility of care. Annals of Internal Medicine. 2003a;138(4):273–287. [PubMed: 12585825]
- Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: Health outcomes and satisfaction with care. Annals of Internal Medicine. 2003b;138(4):288–298. [PubMed: 12585826]
- Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ. Variations in the longitudinal efficiency of academic medical centers, Health Affairs (Millwood). 2004. pp. 19–32. Suppl Web Exclusive:VAR. [PubMed: 15471777]
- IOM (Institute of Medicine). Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press; 2001. [PubMed: 25057539]
- IOM (Institute of Medicine). Performance measurement: Accelerating improvement. Washington, DC: The National Academies Press; 2006.
- IOM (Institute of Medicine). The learning healthcare system: Workshop summary. Washington, DC: The National Academies Press; 2007a. [PubMed: 21452449]
- IOM (Institute of Medicine). Learning what works best: The nation’s need for evidence on comparative effectiveness in health care. 2007b. [accessed January 22, 2008]. http://www
.iom.edu/ebm-effectiveness . - Kessler DP, McClellan M. Do doctors practice defensive medicine? Quarterly Journal of Economics. 1996;111:353–390.
- McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA. The quality of health care delivered to adults in the United States. New England Journal of Medicine. 2003;348(26):2635–2645. [PubMed: 12826639]
- O’Connor AM, Wennberg JE, Legare F, Llewellyn-Thomas HA, Moulton BW, Sepucha KR, Sodano AG, King JS. Toward the “tipping point”: Decision aids and informed patient choice. Health Affairs (Millwood). 2007;26(3):716–725. [PubMed: 17485749]
- O’Connor GT, Plume SK, Olmstead EM, Coffin LH, Morton JR, Maloney CT, Nowicki ER, Tryzelaar JF, Hernandez F, Adrian L. A regional prospective study of in-hospital mortality associated with coronary artery bypass grafting. The Northern New England cardiovascular disease study group. JAMA. 1991;266(6):803–809. [PubMed: 1907669]
- Orszag PR, Ellis P. The challenge of rising health care costs—a view from the Congressional Budget Office. New England Journal of Medicine. 2007;357(18):1793–1795. [PubMed: 17978287]
- Pritchard RS, Fisher ES, Teno JM, Sharp SM, Reding DJ, Knaus WA, Wennberg JE, Lynn J. Influence of patient preferences and local health system characteristics on the place of death. Support investigators. Study to understand prognoses and preferences for risks and outcomes of treatment. Journal of the American Geriatrics Society. 1998;46(10):1242–1250. [PubMed: 9777906]
- Sirovich BE, Gottlieb DJ, Welch HG, Fisher ES. Variation in the tendency of primary care physicians to intervene. Archives of Internal Medicine. 2005;165(19):2252–2256. [PubMed: 16246991]
- Sirovich BE, Gottlieb DJ, Welch HG, Fisher ES. Regional variations in health care intensity and physician perceptions of quality of care. Annals of Internal Medicine. 2006;144(9):641–649. [PubMed: 16670133]
- Skinner JS, Staiger DO, Fisher ES. Is technological change in medicine always worth it? The case of acute myocardial infarction. Health Affairs (Millwood). 2006;25(2):w34–w47. [PMC free article: PMC2117353] [PubMed: 16464904]
- Wennberg JE, Freeman JL, Culp WJ. Are hospital services rationed in new haven or over-utilised in Boston? Lancet. 1987;1(8543):1185–1189. [PubMed: 2883497]
- Wennberg JE, Fisher ES, Skinner JS. Geography and the debate over Medicare reform, Health Affairs (Millwood). 2002. pp. W96–W114. Supp Web Exclusives: [PubMed: 12703563]
- Wennberg JE, Fisher ES, Skinner JS, Bronner KK. Extending the P4P agenda, part 2: How Medicare can reduce waste and improve the care of the chronically ill. Health Affairs. 2007;26(6):1575–1585. [PMC free article: PMC2213719] [PubMed: 17978378]
Footnotes
- 1
The importance of ensuring that care is aligned with patients’ well-informed preferences applies not only to discrete, biologically targeted interventions, but also to care delivery strategies.
- The Need for Better Medical Evidence - Evidence-Based Medicine and the Changing ...The Need for Better Medical Evidence - Evidence-Based Medicine and the Changing Nature of Healthcare
Your browsing activity is empty.
Activity recording is turned off.
See more...