NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009.
4.1. INTRODUCTION
The conditioned place preference paradigm is a standard preclinical behavioral model used to study the rewarding and aversive effects of drugs. Although a number of different designs and apparatuses are used in this model, the basic characteristics of this task involve the association of a particular environment with drug treatment, followed by the association of a different environment with the absence of the drug (i.e., the drug’s vehicle). A common variation of this design consists of a three-compartment chamber with the outer compartments being designed to have different characteristics (e.g., white vs. black walls, pine vs. corn bedding, horizontal grid vs. cross-grid flooring). The center compartment has no special characteristics and is not paired with a drug, and the gates between the compartments can be opened to allow an animal to pass freely between them. During training, an animal (typically a rat or mouse) is given an injection of a drug with potentially rewarding or aversive properties, and is then placed into one of the outer compartments for several minutes. On the following day, the rat is injected with the drug’s vehicle and then placed in the opposite compartment. Generally, these daily sessions alternate between drug and vehicle for 2 or 3 days each. Afterward, a test session is conducted, which consists of placing the animal in the center compartment and then, after opening the gates to both of the outer compartments, recording the time the animal spends in each of the outer compartments during the session. A conditioned place preference (CPP) is found if the animals spend significantly more time in the drug-paired compartment versus the vehicle-paired compartment. On the other hand, if the animals spend significantly more time in the vehicle-paired compartment versus the drug-paired compartment, then this is considered a conditioned place aversion (CPA). Typically, drugs of abuse, such as cocaine, produce CPP, and drugs that elicit aversive effects, such as lithium chloride, produce CPA. As with other behavioral models used in pharmacology research, the behavioral effects of drugs used in the CPP paradigm depend on species, strain, route of administration, time interval of drug administration, dose concentration, and the CPP apparatus used. Many drugs of abuse produce both CPP and CPA, depending on the dose administered. In drug-dependent animals, withdrawal effects generally produce CPA. Because the CPP paradigm generally provides a reliable indicator for studying the rewarding effects of drugs that require relatively little training compared to self-administration paradigm, the CPP paradigm has been commonly used in conjunction with standard neuroscience techniques to elucidate the subjective effects of drugs (Table 4.1).
TABLE 4.1
Common Neuroscience Techniques Used in Conditioned Place Preference Research
4.2. RESEARCH DESIGN AND METHODOLOGICAL CONSIDERATIONS
Although this chapter focuses primarily on studying the effects of drugs of abuse in the CPP model, CPP has also been established with food, copulatory activity, and other rewarding stimuli. The ability of a stimulus, whether it be a drug, food, etc., to produce a preference for the associated environment is generally considered a process governed by Pavlovian, i.e., classical or respondent, conditioning. Using the rewarding effects of a drug as an example, repeatedly pairing the rewarding effects of the drug (unconditioned stimulus, using Pavlov’s terminology) with certain stimuli (i.e., those contained within an environment) would be expected to result in the extension of these rewarding effects to the properties of these previously neutral stimuli. Thus, the drug-paired environment eventually serves as a conditioned stimulus (CS). Although it is not known if the compartment would actually serve as a CS in its truest sense (i.e., elicit rewarding effects similar to those produced by the drug of abuse), dopamine (DA) levels in the nucleus accumbens have been found to be elevated when rats are placed in the drug-paired environment, compared to the nondrug-paired environment [1].
Although the three-compartment chamber described above is a common apparatus used in CPP research (Figure 4.1), other apparatuses vary from this design by having a different number of compartments (e.g., two or four compartments), assessing place preference within an open field, or allowing for the association of the interoceptive effects of drugs with a unique environment. Although all of these approaches have been used to study CPP, an important consideration in the choice of an apparatus is the decision to have a “forced choice” (i.e., the animal must choose the drug-paired side or the nondrug-paired side) or an “unforced choice” (i.e., the animal can remain in a compartment or other area of the apparatus that has not been associated with drug or vehicle) (Figure 4.2, top panel). Thus, a two-compartment apparatus would require a forced choice, whereas a three-compartment area could offer a central choice area between the experimental chambers. Although commonly used, the central concern of using a forced choice procedure is the potential of a bias for the compartment the animal was placed in during the test session.

FIGURE 4.1
Standard two- (left) and three-chamber (right) shuttle boxes used to study conditioned place preference in rodents. Source: From Med-Associates, Inc., with permission.
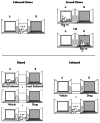
FIGURE 4.2
Unforced versus forced choice procedures (top panel) and biased versus unbiased designs (bottom panel) used in the conditioned place preference paradigm. In an unforced choice procedure, the subject is placed in a central choice area between the parts (more...)
Another important consideration in CPP research is the use of biased versus unbiased research designs. These research designs are used to take into consideration the fact that subjects may have an initial preference for a particular compartment of the apparatus. For instance, if subjects were assessed for place preference in a two-compartment apparatus prior to conditioning, some subjects would spend more time in compartment A, whereas other subjects would spend more time in compartment B. In an unbiased CPP study, the assignment of a particular compartment for pairing with a drug is determined by the researcher, regardless of the preference of each subject for either compartment prior to conditioning (see Figure 4.2, bottom panel). In a biased design, the preference of each individual subject for a particular environment prior to conditioning is assessed first by placing the animals in the apparatus, and then by assessing the amount of time the subjects spend in each compartment. The least-preferred compartment for each subject is then assigned to be the drug-paired compartment. Depending on the design used in a CPP study, different results may occur. For example, early CPP [2] studies with nicotine found discrepant findings between laboratories, which included no effect, CPP, or CPA. In an attempt to clarify these discrepancies, Calcagnetti and Schechter [2] tested nicotine for CPP by first assessing the most and least preferred sides of a three-chamber shuttle box. After baseline preferences were assessed, half of the rats were assigned nicotine for the least preferred side and the remaining half were assigned nicotine for the most preferred side. Nicotine produced a CPP with the least preferred side, but failed to develop a CPP or CPA for the most preferred side (Figure 4.3). Consequently, randomly assigning compartments to be paired with nicotine without assessing baseline preferences may not result in a CPP.

FIGURE 4.3
Effects of nicotine (0.4 mg/kg) in the conditioned place preference paradigm in a biased design with (A) nicotine paired with the most preferred side and (B) nicotine paired with the least preferred side. Baseline preferences for each compartment were (more...)
Other important methodological procedures that should be considered in CPP research include the drug’s time course, the number of conditioning sessions, and the sensory modalities used to discriminate between environments. Generally, drugs that have a slow onset and a long duration of action (e.g., phenobarbital) are not good reinforcers, and consequently, may not readily establish CPPs. For drugs that have potent rewarding properties, fewer conditioning sessions will be needed to establish a CPP (e.g., amphetamine), whereas drugs with weaker rewarding properties may require more conditioning sessions (e.g., nicotine). Finally, sensory modalities should be appropriate for the species being used. For example, visual cues are a poor choice for albino rats, whereas olfactory cues are an excellent choice for these rats. Tactile and auditory cues are also good choices when using rodents.
4.3. TRAINING AND TESTING
Several days of free access to all environments allows the animal to habituate to the apparatus, eliminating novelty as a confounding variable. Baseline data should be determined as an average amount of time spent in each chamber over 3–5 days. The length of time necessary to determine baselines depends on the environmental differences between apparatus chambers. Generally, distinct environments require fewer baseline sessions, whereas less distinct or ambiguous environments require more baseline sessions.
The testing of subjects is performed in a non-manipulated state. The percentage of time spent in each chamber is tabulated during the test session. If the animal spends more time in the chamber associated with treatment, the researcher can infer that the experimental manipulation had a rewarding effect on the affective state of the animal. If the opposite were to occur, then the researcher can infer that the experimental manipulation had an aversive effect on the affective state of the animal. Designs that include a novel chamber (e.g., the center chamber of a three-chamber shuttle box) do not allow free access to the novel chamber during the habituation period.
4.4. DRUG STUDIES USING THE CONDITIONED PLACE PREFERENCE PARADIGM
The CPP paradigm has been widely used in pharmacology, behavioral science, and neuroscience research. A recent database search of Pubmed (http://www.pubmed.gov) using the keywords “conditioned place preference” yielded 1398 results. The CPP paradigm has not simply been used as a screening tool for drug abuse potential, but has been used to study neurotransmitters, brain areas, genes, signaling pathways, and other mechanisms mediating the rewarding (or aversive) effects of drugs. The drugs studied in CPP have been the subject of many reviews [3,4]. Generally, psychostimulants and opiates reliably produce a CPP in this paradigm. For example, systemic administration of cocaine, amphetamine, and nicotine have been found to produce a CPP after two or three pairings in rats and mice [5–14]. In addition, CPP has been established with opiates, such as morphine, heroin, and buprenorphine, as well as drugs from other classes, including the CNS depressants ethanol and diazepam, the cannabinoid receptor agonist delta-9-tetrahydrocannabinal (THC), and the adrenoceptor agonist clonidine [15–19,8,20–32]. Many of these compounds also produce CPA, depending on the dose administered. For example, nicotine produces CPP in rats that are administered doses between 0.4 and 0.8 mg/kg., whereas higher doses are reported to produce CPA [5,33]. Similar findings have been shown with other drugs, such as morphine and the psychostimulant apomorphine [34–39].
4.5. THE MESOLIMBIC DOPAMINE SYSTEM IS IMPORTANT FOR CONDITIONED PLACE PREFERENCE
Although these drugs differ in their CNS effects, the majority of CPP-producing drugs affect the mesolimbic DA system, which consists of DA pathways that originate in the ventral tegmental area (the A10 region in rodents) and terminate in limbic system structures, including the nucleus accumbens and hippocampus. Therefore, DA D2 receptor antagonists, such as haloperidol and metoclopramide, have been found to block CPP or CPA produced by systemically administered amphetamine, cocaine, morphine, and heroin [40–45]. Moreover, direct injection of psychostimulants and opiates into the ventral tegmental area or the nucleus accumbens also produces CPP, whereas direct injection of amphetamine or morphine into other areas, such as the prefrontal cortex, caudate, or amygdala, fails to produce CPP or CPA [46–52]. In rats that developed a CPP for cocaine after conditioning, significantly greater elevations in DA levels were found in the nucleus accumbens after vehicle injection when rats were placed in the cocaine-paired compartment, as opposed to the vehicle-paired compartment [53,54]. However, DA levels in the prefrontal cortex have also been found to be elevated in rats placed in an amphetamine-paired compartment after several days of conditioning, and selective depletion of prefrontal cortical norepinephrine prevents amphetamine- and morphine-induced CPP and amphetamine- and morphine-induced DA release in the nucleus accumbens [1,55,56]. Selective lesioning of DA terminals using 6-hydroxydopamine in the ventral pallidum, another target region of mesolimbic DA neurons, has been shown to attenuate the development of cocaine-induced CPP [57]. CPP has also resulted from morphine infusion into the hippocampus [58]. Thus, although the nucleus accumbens is an important region that mediates the effects of drugs of abuse, other limbic structures, as well as structures that mediate limbic system function, may alter the ability of drugs of abuse to elicit CPP.
4.6. MECHANISMS MEDIATING CONDITIONED PLACE PREFERENCE FOR COMMON DRUGS OF ABUSE
4.6.1. Opiates
As presented earlier, opiates, such as morphine, are capable of producing CPP in rats and mice. The ability of opiate drugs to elicit a CPP depends, in part, on the release of dopamine from mesolimbic DA neurons, because morphine- and heroin-induced CPP can be blocked by the DA D2 receptor antagonists haloperidol and metoclopramide. Furthermore, direct injection of opiates into the ventral tegmental area or nucleus accumbens also produces CPP [40,42,46–48,49,51]. Opiates are known to enhance mesolimbic DA neuronal firing and ultimately release DA into the nucleus accumbens, which has been shown to be caused by a disinhibition of DA neurons in the ventral tegmental area through attenuating gamma-aminobutyric acid (GABA) release in the ventral tegmental area. The μ-opioid receptor has long been identified as the key receptor for mediating the subjective effects of opiates, and is also likely responsible for mediating opiate-induced CPP. The opioid receptor partial agonist buprenorphine has been shown to produce CPP and increase locomotor activity in wild type (WT) mice, but not in μ-receptor knockout (KO) mutant mice; whereas amphetamine has been shown to produce CPP and increase locomotor activity in both WT and μ KO mice [31]. Moreover, agonists selective for μ-opioid receptors can reinstate CPP in formerly morphine-dependent rats trained in the CPP procedure [59].
Other neurotransmitters may also be important for the development of CPP by opiates. CPP produced by morphine has been shown to be attenuated after pre-treatment with the cannabinoid CB1 receptor antagonist SR-141716 [60]. In addition to potential modulation of morphine-induced CPP by cannabinoid receptors, intraventral tegmental area infusions of nicotine and the acetylcholinesterase inhibitor neostigmine have been found to facilitate CPP produced by an otherwise ineffective dose (0.5 mg/kg) of morphine. Intraventral tegmental area infusions of the muscarinic receptor antagonist atropine and the nicotinic receptor antagonist mecamylamine have been found to prevent CPP produced by an effective dose of morphine (5.0 mg/kg), suggesting that the cholinergic system may also mediate the rewarding effects of morphine and other opiates [32]. Local administration of the glutamate ion channel agonist N-methyl-D-aspartate (NMDA) into the amygdala has been shown to potentiate morphine-induced CPP, whereas the glutamate ion channel blocker MK-801 has been shown to attenuate morphine-induced CPP [61].
4.6.2. Psychostimulants
As noted above, psychostimulants such as amphetamine and cocaine often produce a robust CPP, and these effects also depend upon limbic system functioning, particularly on the release of DA into the nucleus accumbens. Although psychostimulants as a class tend to produce rewarding effects, as demonstrated in CPP and self-administration studies, drugs in this class vary greatly as to the mechanisms mediating reward. Apomorphine, a psychostimulant and classic DA receptor agonist, has been reported to produce CPP in numerous studies, as well as CPA at relatively high doses [34,41,62,63]. The rewarding effects of apomorphine are potentiated in the CPP model by pretreatment with the DA D3 receptor agonist 7-OH-DPAT, yet in the same study pretreatment with 7-OH-DPAT was found to attenuate the rewarding effects of cocaine [63]. Moreover, systemic administration of the typical antipsychotic drug and D2 receptor antagonist haloperidol, as well as 6-OH-DA lesions in the nucleus accumbens, failed to attenuate cocaine-induced CPP [64]. However, another study reported that haloperidol did prevent cocaine-induced CPP when cocaine was administered intravenously [44]. The D1 receptor antagonist SCH23390 also has been shown to block cocaine-induced CPP in both male and female rats [65]. Cocaine, unlike apomorphine and other psychostimulants, with the exception of amphetamine, which is a competitor with DA for the vesicular DA transporter, is an inhibitor of DA, norepinephrine, and serotonin transporters; although the dopamine transporter (DAT) inhibition is generally thought to be most important for the rewarding effects of cocaine. However, DAT KO mice exhibit a CPP for cocaine and are still found to self-administer cocaine [66,67]. However, cocaine-induced CPP in DAT KO mice may be due to compensatory changes in DA systems in the DAT KO mice, given that cocaine-induced CPP is not found in a triple mutant DAT KO mouse line that results in a relatively cocaine-insensitive DAT that still transports DA [68]. Amphetamine-induced CPP is also abolished in DAT KO mice, but can be blocked by haloperidol [4,41,43]. The rewarding effects of amphetamine may be mediated, at least in part, by serotonin receptors, given that the amphetamine-induced CPP is also blocked by the 5-HT2A/2B/2C receptor antagonist ritanserin and by the 5-HT reuptake inhibitors zemilidine and fluoxetine [69–71]. Despite differences between cocaine and amphetamine in the CPP paradigm, both cocaine and amphetamine have been reported to elevate cocaine- and amphetamine-regulated transcript (CART) mRNA levels after acute administration in the nucleus accumbens and ventral tegmental area, and bilateral intraventral tegmental area injections of the CART peptide fragment 55-102 have been found to produce CPP [72–74]. Moreover, CART 55-102–induced CPP was blocked by systemic administration of haloperidol.
4.6.3. Nicotine
Systemic administration of nicotine has been shown to produce both CPP and CPA in rodents through stimulation of nicotinic acetylcholine receptors (nAChrs) [5,75,76]. Both CPP and CPA is observed at low and high doses, respectively, after intra-ventral tegmental area infusions of nicotine, which can be blocked by both the α4β2 nAChr antagonist DHbeteE and the α7 nAChr antagonist methyllycaconitine (MLA) [52]. Moreover, MLA pretreatment shifted nicotine-induced CPP to CPA in this study. In nicotine-dependent rats, pairing withdrawal effects induced by administration of the nonselective nAChr antagonist mecamylamine produces CPA, which can be prevented upon coadministration of the 5-HT3 receptor antagonist ondansetron [77]. The nAChr antagonist epibatidine produces a relatively weak CPP when administered systemically alone, but also produces a CPA at higher doses, and further nAChr studies have revealed that β2, but not α7, nAChrs are necessary to establish nicotine-induced CPP [78,79].
The ability of nicotine to produce CPP differs markedly between strains of rats. For example, nicotine CPP has been established in the Lewis strain of rat, but not in the Fischer-344 strain of rat [10,13]. Further individual differences in susceptibility to nicotine dependence have been shown within the same strain of mice, in which a single injection of nicotine (0.75 mg/kg) was found to either increase or decrease locomotor activity, and subsequent testing for nicotine CPP found that nicotine produced CPP in mice that had increased locomotor activity after nicotine administration, and that nicotine produced a lower degree of CPP in mice that had decreased locomotor activity after nicotine administration [80].
4.6.4. Ethanol
Ethanol, when administered alone, produces CPP and CPA in rodents, with lower doses producing CPP and higher doses producing CPA [21,24,29,81]. Receptor mechanisms found to mediate ethanol’s CNS effects, GABAA, NMDA, and 5-HT3 receptors, have also been tested to determine which receptors mediate reward using the CPP paradigm. Ethanol-induced CPP was shown to be attenuated when ethanol was coadministered with the competitive NMDA receptor antagonist CGP-37849, but not when coadministered with the noncompetitive NMDA receptor antagonists MK-801 and ketamine, nor with NMDA subunit antagonists [82]. Again, the mesolimbic DA pathway appears critical for the rewarding effects of ethanol, since ethanol-induced CPP can be potentiated by systemically administered heroin, and can be attenuated by intra-accumbens administration of D2 receptor antagonists, such as fluphenazine [83]. Ethanol has been found to potentiate the effects of cocaine in the CPP model by shifting high cocaine doses from producing CPP to CPA and by increasing CPP induced by lower cocaine doses [84,85].
In the liver, ethanol is broken down by alcohol dehydrogenase to acetaldehyde, which in turn is broken down by aldehyde dehydrogenase to acetic acid. When acetaldehyde accumulates, symptoms of acetaldehyde syndrome may occur, which include nausea, headache, and vomiting. In the CPP paradigm, acetaldehyde has been shown to produce CPP, but not CPA, including doses that approached the lethal limit [81]. Intriguingly, deactivation of acetaldehyde by d-penicillamine prevents ethanol-induced CPP, whereas ethanol-induced CPA is unaffected, adding to evidence from other studies that acetaldehyde may mediate the rewarding effects (e.g., euphoria) of ethanol [86–89].
4.6.5. MDMA
3,4-Methylenedioxymethamphetamine (MDMA) has been shown to readily establish a CPP in rodents [90–92]. The ability of MDMA to produce a CPP may be caused by effects on the mesolimbic DA pathways, based on a microdialysis study, which found that doses of MDMA that produced CPPs also significantly elevated levels of DA and lowered levels of the DA metabolite DOPAC in the nucleus accumbens [93]. Moreover, MDMA-induced CPP is attenuated upon pretreatment with the 5-HT3 receptor antagonist MDL72222 and tropisetron [94,95]. MDMA-induced CPP has also been found to be diminished by pretreatment of the cannabinoid CB1 receptor antagonist SR141716A and the opioid antagonist naltrexone [95]. In adolescents, the neurotoxic effects of MDMA appear to be diminished, suggesting that the MDMA receptors become more prominent in later development, perhaps during puberty. In a study by Fone et al. [96], cocaine produced CPP in adolescent rats previously treated for three consecutive days with MDMA, whereas cocaine failed to produce CPP in adolescent rats treated for seven days with the MDMA vehicle. Aberg et al. [97] found a similar effect in adolescent rats, and interestingly, these effects were reversed in adult rats; MDMA-pretreated rats exhibited a diminished CPP for cocaine compared to vehicle pretreated rats. The rewarding effects of MDMA have also been shown to be potentiated when coadministered with delta-9-THC, the principle psychoactive ingredient in cannabis. Robledo et al. [98] found that doses of delta-9-THC (0.3 mg/kg) and MDMA (3.0 mg/kg) that did not produce CPP when administered alone, did produce CPP when coadministered.
4.6.6. Delta-9-THC and Endocannabinoids
Delta-9-THC, the psychoactive ingredient in smoked cannabis, has been shown to produce CPP under certain conditions. Initial findings of THC CPP were reported in rats using doses that ranged from 2.0–4.0 mg/kg, but a study that came out soon after reported that a THC CPP was not demonstrated by a 1.5 mg/kg dose in rats [99,100]. In fact, the later study reported a CPA to THC following a 15 mg/kg dose, and that the cannabinoid CB1 receptor antagonist SR141716A produced a CPP [100]. A CPA to THC has also been found in mice [25,101]. Recently, the rewarding effects of THC in the CPP paradigm have been shown in mice after administering an injection of THC (as a priming dose) 24 hr prior to beginning several daily conditioning sessions with THC. This modified procedure resulted in a CPP for THC [25,102]. In this methodological variation, the initial administration of THC may have resulted in tolerance to the dysphoric, or otherwise aversive, effects of THC, thus enabling the rewarding effects of THC to become more salient during the following conditioning trials with THC. However, the inability of some studies to establish a CPP for THC may have been a result of the doses used, since Braida et al. [103] reported that THC, at doses ranging from 0.075–0.75 mg/kg, produced a CPP in Wistar rats. The opioid system may mediate the effects of THC in the CPP paradigm, because CPP for THC has been shown to be attenuated in μ/δ-opioid receptor double-KO mice, and that chronic administration of THC produces cross-tolerance to the rewarding effects of morphine in the CPP paradigm [102,104].
The endogenous cannabinoid anandamide has not been well characterized in the CPP paradigm, but doses up to 16.0 mg/kg have failed to produce a CPP, despite coadministration of a protease inhibitor to lengthen the half-life of anandamide [105]. However, the anandamide transport inhibitor AM404 was shown to produce a CPP in rats raised in an enriched environment, suggesting that anandamide may be capable of producing a CPP under certain conditions [106].
4.7. CONDITIONED PLACE PREFERENCE VERSUS SELF-ADMINISTRATION
Another common model for assessing the rewarding properties of drugs is the self-administration paradigm. As the name suggests, this paradigm consists of recording the number of times an animal produces a response (e.g., a lever press) that results in an infusion of drug, which is usually given intravenously. The self-administration paradigm is an important tool for screening drugs for abuse potential and to elucidate the rewarding effects of drugs. Although the conditioned place preference and self-administration paradigms both measure the rewarding properties of drugs, there are important differences between these two models (Table 4.2). First, although both CPP and self-administration studies are sensitive to the rewarding effects of many of the same drugs, including psychostimulants and opiates, some drugs produce CPP but may not be self administered (e.g., LSD, buspirone, and pentylenetetrazole), while others are self administered but do not produce CPP (e.g., pentobarbital and phencyclidine) [4]. Second, the preponderance of CPP studies have used only rats and mice, whereas self-administration studies have been conducted in monkeys, rats, mice, and pigeons. Third, the mechanisms that mediate drug-induced CPP and self-administration of a drug may be different. For example, D2 receptor antagonists have minimal effects on the ability of cocaine to produce CPP, whereas D2 antagonists readily attenuate self-administration for cocaine [11]. Finally, an important contrast between these two paradigms is the difference in methodological procedures. Unlike the CPP paradigm, the self-administration paradigm requires surgical implantation of a catheter, usually for intravenous drug administration, and an extensive operant training history. Moreover, in CPP, the subjective effects of the drug are present prior to the task, whereas in the self-administration paradigm, a subject is learning a task where responses produce near-immediate effects from drug administration. The latter appears to be most similar of these two models to drug use in humans.
TABLE 4.2
Comparison of Conditioned Place Preference and Self-Administration
4.8. SUMMARY
The CPP paradigm is a useful tool for studying the affective properties of drugs, and is routinely used in concert with standard research techniques in neuroscience. Most drugs of abuse elicit a CPP in rats and mice, and the neural substrates of these effects can often be traced to the mesolimbic DA system. Alternative models for assessing the rewarding effects of drugs (e.g., self-administration) do not always produce similar results, and therefore, researchers should be careful when evaluating results based on the behavioral model they are using in their study.
ACKNOWLEDGMENTS
The authors wish to thank Med Associates, Inc. for providing the images used in Figure 4.1 and Juan Rodriguez for producing the illustrations used in Figure 4.2.
REFERENCES
- 1.
- Lin SK, Pan WH, Yeh PH. Prefrontal dopamine efflux during exposure to drug-associated contextual cues in rats with prior repeated methamphetamine. Brain Res Bull. 2007;71:365–371. [PubMed: 17208653]
- 2.
- Calcagnetti DJ, Schechter MD. Nicotine place preference using the biased method of conditioning. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1994;18:925–933. [PubMed: 7972862]
- 3.
- Hoffman DC. The use of place conditioning in studying the neuropharmacology of drug reinforcement. Brain Res Bull. 1989;23:373–387. [PubMed: 2556210]
- 4.
- Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl.). 2000;153:31–43. [PubMed: 11255927]
- 5.
- Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22:237–241. [PubMed: 2858867]
- 6.
- Tuazon DB, Suzuki T, Misawa M, Watanabe S. Methylxanthines (caffeine and theophylline) blocked methamphetamine-induced conditioned place preference in mice but enhanced that induced by cocaine. Ann NY Acad Sci. 1992;654:531–533. [PubMed: 1632619]
- 7.
- Masukawa Y, Suzuki T, Misawa M. Differential modification of the rewarding effects of methamphetamine and cocaine by opioids and antihistamines. Psychopharmacology (Berl.). 1993;111:139–143. [PubMed: 7870944]
- 8.
- Suzuki T, Misawa M. Sertindole antagonizes morphine-, cocaine-, and methamphetamine-induced place preference in the rat. Life Sci. 1995;57:1277–1284. [PubMed: 7674819]
- 9.
- Suzuki T, Sugano Y, Funada M, Misawa M. Adrenalectomy potentiates the morphine—but not cocaine-induced place preference in rats. Life Sci. 1995;56:PL339–PL344. [PubMed: 8847945]
- 10.
- Horan B, Smith M, Gardner EL, Lepore M, Ashby CR Jr. (−)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse. 1997;26:93–94. [PubMed: 9097409]
- 11.
- Bardo MT, Valone JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: Role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology (Berl.). 1999;143:39–46. [PubMed: 10227078]
- 12.
- Busse GD, Riley AL. Cocaine, but not alcohol, reinstates cocaine-induced place preferences. Pharmacol Biochem Behav. 2004;78:827–833. [PubMed: 15301942]
- 13.
- Philibin SD, Vann RE, Varvel SA, et al. Differential behavioral responses to nicotine in Lewis and Fischer-344 rats. Pharmacol Biochem Behav. 2005;80:87–92. [PubMed: 15652384]
- 14.
- Ettenberg A, Bernardi RE. Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;87:171–178. [PMC free article: PMC1949322] [PubMed: 17524462]
- 15.
- Asin KE, Wirtshafter D. Clonidine produces a conditioned place preference in rats. Psychopharmacology (Berl.). 1985;85:383–385. [PubMed: 3923526]
- 16.
- Brown EE, Finlay JM, Wong JT, Damsma G, Fibiger HC. Behavioral and neurochemical interactions between cocaine and buprenorphine: Implications for the pharmacotherapy of cocaine abuse. J Pharmacol Exp Ther. 1991;256:119–126. [PubMed: 1988653]
- 17.
- Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. [PubMed: 1328733]
- 18.
- Cervo L, Rossi C, Samanin R. Clonidine-induced place preference is mediated by alpha 2-adrenoceptors outside the locus coeruleus. Eur J Pharmacol. 1993;238:201–207. [PubMed: 8104806]
- 19.
- Suzuki T, Funada M, Narita M, Misawa M, Nagase H. Morphine-induced place preference in the CXBK mouse: Characteristics of mu opioid receptor subtypes. Brain Res. 1993;602:45–52. [PubMed: 8383571]
- 20.
- Gaiardi M, Bartoletti M, Bacchi A, Gubellini C, Babbini M. Motivational properties of buprenorphine as assessed by place and taste conditioning in rats. Psychopharmacology (Berl.). 1997;130:104–108. [PubMed: 9106906]
- 21.
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Involvement of dopamine D(1) and D(2) receptors in the ethanol-associated place preference in rats exposed to conditioned fear stress. Brain Res. 1999;835:298–305. [PubMed: 10415386]
- 22.
- McFarland K, Ettenberg A. Haloperidol does not attenuate conditioned place preferences or locomotor activation produced by food- or heroin-predictive discriminative cues. Pharmacol Biochem Behav. 1999;62:631–641. [PubMed: 10208369]
- 23.
- Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: A conditioned place preference study. Psychopharmacology (Berl.). 2000;151:25–30. [PubMed: 10958113]
- 24.
- Matsuzawa S, Suzuki T, Misawa M. Ethanol, but not the anxiolytic drugs buspirone and diazepam, produces a conditioned place preference in rats exposed to conditioned fear stress. Pharmacol Biochem Behav. 2000;65:281–288. [PubMed: 10672981]
- 25.
- Valjent E, Maldonado R. A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl.). 2000;147:436–438. [PubMed: 10672638]
- 26.
- Braida D, Pozzi M, Cavallini R, Sala M. Conditioned place preference induced by the cannabinoid agonist CP 55,940: Interaction with the opioid system. Neuroscience. 2001;104:923–926. [PubMed: 11457579]
- 27.
- Robinson L, Hinder L, Pertwee RG, Riedel G. Effects of delta9-THC and WIN-55,212-2 on place preference in the water maze in rats. Psychopharmacology (Berl.). 2003;166:40–50. [PubMed: 12488948]
- 28.
- Walker BM, Ettenberg A. The effects of alprazolam on conditioned place preferences produced by intravenous heroin. Pharmacol Biochem Behav. 2003;75:75–80. [PubMed: 12759115]
- 29.
- Busse GD, Lawrence ET, Riley AL. The effects of alcohol preexposure on cocaine, alcohol and cocaine/alcohol place conditioning. Pharmacol Biochem Behav. 2005;81:459–465. [PubMed: 15907338]
- 30.
- Simpson GR, Riley AL. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmacol Biochem Behav. 2005;80:471–479. [PubMed: 15740790]
- 31.
- Marquez P, Baliram R, Kieffer BL, Lutfy K. The mu opioid receptor is involved in buprenorphine-induced locomotor stimulation and conditioned place preference. Neuropharmacology. 2007;52:1336–1341. [PMC free article: PMC2267901] [PubMed: 17367825]
- 32.
- Rezayof A, Nazari-Serenjeh F, Zarrindast MR, Sepehri H, Delphi L. Morphine-induced place preference: Involvement of cholinergic receptors of the ventral tegmental area. Eur J Pharmacol. 2007;562:92–102. [PubMed: 17336285]
- 33.
- Le Foll B, Goldberg SR. Control of the reinforcing effects of nicotine by associated environmental stimuli in animals and humans. Trends Pharmacol Sci. 2005;26:287–293. [PubMed: 15925703]
- 34.
- Best PJ, Best MR, Mickley GA. Conditioned aversion to distinct environmental stimuli resulting from gastrointestinal distress. J Comp Physiol Psychol. 1973;85:250–257. [PubMed: 4756903]
- 35.
- van der Kooy D, Swerdlow NR, Koob GF. Paradoxical reinforcing properties of apomorphine: Effects of nucleus accumbens and area postrema lesions. Brain Res. 1983;259:111–118. [PubMed: 6824923]
- 36.
- Bechara A, van der Kooy D. Opposite motivational effects of endogenous opioids in brain and periphery. Nature. 1985;314:533–534. [PubMed: 2986002]
- 37.
- Bechara A, Zito KA, van der Kooy D. Peripheral receptors mediate the aversive conditioning effects of morphine in the rat. Pharmacol Biochem Behav. 1987;28:219–225. [PubMed: 2825220]
- 38.
- Papp M. Different effects of short- and long-term treatment with imipramine on the apomorphine- and food-induced place preference conditioning in rats. Pharmacol Biochem Behav. 1988;30:889–893. [PubMed: 3227037]
- 39.
- Zito KA, Bechara A, Greenwood C, van der Kooy D. The dopamine innervation of the visceral cortex mediates the aversive effects of opiates. Pharmacol Biochem Behav. 1988;30:693–699. [PubMed: 3211979]
- 40.
- Schwartz AS, Marchok PL. Depression of morphine-seeking behaviour by dopamine inhibition. Nature. 1974;248:257–258. [PubMed: 4274273]
- 41.
- Spyraki C, Fibiger HC, Phillips AG. Dopaminergic substrates of amphetamine-induced place preference conditioning. Brain Res. 1982;253:185–193. [PubMed: 6817850]
- 42.
- Spyraki C, Fibiger HC, Phillips AG. Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system. Psychopharmacology (Berl.). 1983;79:278–283. [PubMed: 6405439]
- 43.
- Mithani S, Martin-Iverson MT, Phillips AG, Fibiger HC. The effects of haloperidol on amphetamine- and methylphenidate-induced conditioned place preferences and locomotor activity. Psychopharmacology (Berl.). 1986;90:247–252. [PubMed: 3097706]
- 44.
- Spyraki C, Nomikos GG, Varonos DD. Intravenous cocaine-induced place preference: Attenuation by haloperidol. Behav Brain Res. 1987;26:57–62. [PubMed: 3675835]
- 45.
- Hoffman DC, Beninger RJ. The effects of selective dopamine D1 or D2 receptor antagonists on the establishment of agonist-induced place conditioning in rats. Pharmacol Biochem Behav. 1989;33:273–279. [PubMed: 2573075]
- 46.
- Phillips AG, LePiane FG. Reinforcing effects of morphine microinjection into the ventral tegmental area. Pharmacol Biochem Behav. 1980;12:965–968. [PubMed: 7403209]
- 47.
- van der Kooy D, Mucha RF, O’Shaughnessy M, Bucenieks P. Reinforcing effects of brain microinjections of morphine revealed by conditioned place preference. Brain Res. 1982;243:107–117. [PubMed: 7116146]
- 48.
- Phillips AG, LePiane FG, Fibiger HC. Dopaminergic mediation of reward produced by direct injection of enkephalin into the ventral tegmental area of the rat. Life Sci. 1983;33:2505–2511. [PubMed: 6417434]
- 49.
- Glimcher PW, Giovino AA, Margolin DH, Hoebel BG. Endogenous opiate reward induced by an enkephalinase inhibitor, thiorphan, injected into the ventral midbrain. Behav Neurosci. 1984;98:262–268. [PubMed: 6586195]
- 50.
- Carr GD, White NM. Anatomical disassociation of amphetamine’s rewarding and aversive effects: An intracranial microinjection study. Psychopharmacology (Berl.). 1986;89:340–346. [PubMed: 3088661]
- 51.
- Bozarth MA. Neuroanatomical boundaries of the reward-relevant opiate-receptor field in the ventral tegmental area as mapped by the conditioned place preference method in rats. Brain Res. 1987;414:77–84. [PubMed: 3620924]
- 52.
- Laviolette SR, van der Kooy D. The motivational valence of nicotine in the rat ventral tegmental area is switched from rewarding to aversive following blockade of the alpha7-subunit-containing nicotinic acetylcholine receptor. Psychopharmacology (Berl.). 2003;166:306–313. [PubMed: 12569428]
- 53.
- Duvauchelle CL, Ikegami A, Asami S, Robens J, Kressin K, Castaneda E. Effects of cocaine context on NAcc dopamine and behavioral activity after repeated intravenous cocaine administration. Brain Res. 2000;862:49–58. [PubMed: 10799668]
- 54.
- Duvauchelle CL, Ikegami A, Castaneda E. Conditioned increases in behavioral activity and accumbens dopamine levels produced by intravenous cocaine. Behav Neurosci. 2000;114:1156–1166. [PubMed: 11142647]
- 55.
- Ventura R, Cabib S, Alcaro A, Orsini C, Puglisi-Allegra S. Norepinephrine in the prefrontal cortex is critical for amphetamine-induced reward and mesoaccumbens dopamine release. J Neurosci. 2003;23:1879–1885. [PMC free article: PMC6741949] [PubMed: 12629192]
- 56.
- Ventura R, Alcaro A, Puglisi-Allegra S. Prefrontal cortical norepinephrine release is critical for morphine-induced reward, reinstatement and dopamine release in the nucleus accumbens. Cereb. Cortex. 2005;15:1877–1886. [PubMed: 15728739]
- 57.
- Gong W, Neill D, Justice JB Jr. 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res. 1997;754:103–112. [PubMed: 9134965]
- 58.
- Corrigall WA, Linseman MA. Conditioned place preference produced by intra-hippocampal morphine. Pharmacol Biochem Behav. 1988;30:787–789. [PubMed: 3211988]
- 59.
- Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur J Pharmacol. 2007;566:75–82. [PubMed: 17383633]
- 60.
- Singh ME, Verty AN, McGregor IS, Mallet PE. A cannabinoid receptor antagonist attenuates conditioned place preference but not behavioural sensitization to morphine. Brain Res. 2004;1026:244–253. [PubMed: 15488486]
- 61.
- Rezayof A, Golhasani-Keshtan F, Haeri-Rohani A, Zarrindast MR. Morphine-induced place preference: Involvement of the central amygdala NMDA receptors. Brain Res. 2007;1133:34–41. [PubMed: 17184750]
- 62.
- Swerdlow NR, Swanson LW, Koob GF. Electrolytic lesions of the substantia innominata and lateral preoptic area attenuate the “supersensitive” locomotor response to apomorphine resulting from denervation of the nucleus accumbens. Brain Res. 1984;306:141–148. [PubMed: 6087974]
- 63.
- Khroyan TV, Fuchs RA, Beck AM, Groff RS, Neisewander JL. Behavioral interactions produced by co-administration of 7-OH-DPAT with cocaine or apomorphine in the rat. Psychopharmacology (Berl.). 1999;142:383–392. [PubMed: 10229063]
- 64.
- Spyraki C, Fibiger HC, Phillips AG. Cocaine-induced place preference conditioning: Lack of effects of neuroleptics and 6-hydroxydopamine lesions. Brain Res. 1982;253:195–203. [PubMed: 6817851]
- 65.
- Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V. The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats. Brain Res Bull. 2004;63:295–299. [PubMed: 15196654]
- 66.
- Sora I, Wichems C, Takahashi N, et al. Cocaine reward models: Conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc. Natl. Acad. Sci. USA. 1998;95:7699–7704. [PMC free article: PMC22727] [PubMed: 9636213]
- 67.
- Medvedev IO, Gainetdinov RR, Sotnikova TD, Bohn LM, Caron MG, Dykstra LA. Characterization of conditioned place preference to cocaine in congenic dopamine transporter knockout female mice. Psychopharmacology (Berl.). 2005;180:408–413. [PubMed: 15719221]
- 68.
- Chen R, Tilley MR, Wei H, et al. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc. Natl. Acad. Sci. USA. 2006;103:9333–9338. [PMC free article: PMC1482610] [PubMed: 16754872]
- 69.
- Kruszewska A, Romandini S, Samanin R. Different effects of zimelidine on the reinforcing properties of d-amphetamine and morphine on conditioned place preference in rats. Eur J Pharmacol. 1986;125:283–286. [PubMed: 2943599]
- 70.
- Nomikos GG, Spyraki C. Effects of ritanserin on the rewarding properties of d-amphetamine, morphine and diazepam revealed by conditioned place preference in rats. Pharmacol Biochem Behav. 1988;30:853–858. [PubMed: 3147460]
- 71.
- Takamatsu Y, Yamamoto H, Ogai Y, Hagino Y, Markou A, Ikeda K. Fluoxetine as a potential pharmacotherapy for methamphetamine dependence: Studies in mice. Ann NY Acad Sci. 2006;1074:295–302. [PubMed: 17105925]
- 72.
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. [PMC free article: PMC6578117] [PubMed: 7891182]
- 73.
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed: 9527537]
- 74.
- Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intraventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55-102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–792. [PubMed: 10900261]
- 75.
- Fudala PJ, Iwamoto ET. Further studies on nicotine-induced conditioned place preference in the rat. Pharmacol Biochem Behav. 1986;25:1041–1049. [PubMed: 3786357]
- 76.
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl.). 2005;178:481–492. [PubMed: 15765262]
- 77.
- Suzuki T, Ise Y, Mori T, Misawa M. Attenuation of mecamylamine-precipitated nicotine-withdrawal aversion by the 5-HT3 receptor antagonist ondansetron. Life Sci. 1997;61:PL249–PL254. [PubMed: 9353175]
- 78.
- Janhunen S, Linnervuo A, Svensk M, Ahtee L. Effects of nicotine and epibatidine on locomotor activity and conditioned place preference in rats. Pharmacol Biochem Behav. 2005;82:758–765. [PubMed: 16413603]
- 79.
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl.). 2006;184:339–344. [PubMed: 16416156]
- 80.
- Schechter MD, Meehan SM, Schechter JB. Genetic selection for nicotine activity in mice correlates with conditioned place preference. Eur J Pharmacol. 1995;279:59–64. [PubMed: 7556383]
- 81.
- Quertemont E, De Witte P. Conditioned stimulus preference after acetaldehyde but not ethanol injections. Pharmacol Biochem Behav. 2001;68:449–454. [PubMed: 11325398]
- 82.
- Boyce-Rustay JM, Cunningham CL. The role of NMDA receptor binding sites in ethanol place conditioning. Behav Neurosci. 2004;118:822–834. [PubMed: 15301608]
- 83.
- Walker BM, Ettenberg A. Intracerebroventricular ethanol-induced conditioned place preferences are prevented by fluphenazine infusions into the nucleus accumbens of rats. Behav Neurosci. 2007;121:401–410. [PubMed: 17469930]
- 84.
- Busse GD, Riley AL. Modulation of cocaine-induced place preferences by alcohol. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:1373–1381. [PubMed: 12502026]
- 85.
- Busse GD, Lawrence ET, Riley AL. The modulation of cocaine-induced conditioned place preferences by alcohol: effects of cocaine dose. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:149–155. [PubMed: 14687869]
- 86.
- Brown ZW, Amit Z, Rockman GE. Intraventricular self-administration of acetaldehyde, but not ethanol, in naive laboratory rats. Psychopharmacology (Berl.). 1979;64:271–276. [PubMed: 41277]
- 87.
- Smith BR, Amit Z, Splawinsky J. Conditioned place preference induced by intraventricular infusions of acetaldehyde. Alcohol. 1984;1:193–195. [PubMed: 6536284]
- 88.
- Rodd ZA, Bell RL, Zhang Y, et al. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: Involvement of dopamine and serotonin. Neuropsychopharmacology. 2005;30:330–338. [PubMed: 15383830]
- 89.
- Font L, Aragon CM, Miquel M. Ethanol-induced conditioned place preference, but not aversion, is blocked by treatment with D-penicillamine, an inactivation agent for acetaldehyde. Psychopharmacology (Berl.). 2006;184:56–64. [PubMed: 16344987]
- 90.
- Bilsky EJ, Hui YZ, Hubbell CL, Reid LD. Methylenedioxymethamphetamine’s capacity to establish place preferences and modify intake of an alcoholic beverage. Pharmacol Biochem Behav. 1990;37:633–638. [PubMed: 1982692]
- 91.
- Bilsky EJ, Hubbell CL, Delconte JD, Reid LD. MDMA produces a conditioned place preference and elicits ejaculation in male rats: A modulatory role for the endogenous opioids. Pharmacol Biochem Behav. 1991;40:443–447. [PubMed: 1687169]
- 92.
- Daza-Losada M, Ribeiro Do Couto B, Manzanedo C, Aguilar MA, Rodriguez-Arias M, Minarro J. Rewarding effects and reinstatement of MDMA-induced CPP in adolescent mice. Neuropsychopharmacology. 2007;32:1750–1759. [PubMed: 17299518]
- 93.
- Marona-Lewicka D, Rhee GS, Sprague JE, Nichols DE. Reinforcing effects of certain serotonin-releasing amphetamine derivatives. Pharmacol Biochem Behav. 1996;53:99–105. [PubMed: 8848466]
- 94.
- Bilsky EJ, Reid LD. MDL72222, a serotonin 5-HT3 receptor antagonist, blocks MDMA’s ability to establish a conditioned place preference. Pharmacol Biochem Behav. 1991;39:509–512. [PubMed: 1682951]
- 95.
- Braida D, Iosue S, Pegorini S, Sala M. 3, 4 Methylenedioxymethamphetamine-induced conditioned place preference (CPP) is mediated by endocannabinoid system. Pharmacol Res. 2005;51:177–182. [PubMed: 15629265]
- 96.
- Fone KC, Beckett SR, Topham IA, Swettenham J, Ball M, Maddocks L. Long-term changes in social interaction and reward following repeated MDMA administration to adolescent rats without accompanying serotonergic neurotoxicity. Psychopharmacology (Berl.). 2002;159:437–444. [PubMed: 11823897]
- 97.
- Aberg M, Wade D, Wall E, Izenwasser S. Effect of MDMA (ecstasy) on activity and cocaine conditioned place preference in adult and adolescent rats. Neurotoxicol Teratol. 2007;29:37–46. [PMC free article: PMC1817672] [PubMed: 17049207]
- 98.
- Robledo P, Trigo JM, Panayi F, de la Torre R, Maldonado R. Behavioural and neurochemical effects of combined MDMA and THC administration in mice. Psychopharmacology (Berl). 2007;195:255–264. [PubMed: 17684733]
- 99.
- Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by delta 9-tetrahydrocannabinol: Comparison with cocaine, morphine, and food reward. Life Sci. 1995;56:2073–2080. [PubMed: 7776834]
- 100.
- Sanudo-Pena MC, Tsou K, Delay ER, Hohman AG, Force M, Walker JM. Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci Lett. 1997;223:125–128. [PubMed: 9089689]
- 101.
- Hutcheson DM, Tzavara ET, Smadja C, et al. Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9-tetrahydrocannabinol. Br J Pharmacol. 1998;125:1567–1577. [PMC free article: PMC1565737] [PubMed: 9884086]
- 102.
- Castane A, Robledo P, Matifas A, Kieffer BL, Maldonado R. Cannabinoid withdrawal syndrome is reduced in double mu and delta opioid receptor knockout mice. Eur J Neurosci. 2003;17:155–159. [PubMed: 12534979]
- 103.
- Braida D, Iosue S, Pegorini S, Sala M. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004;506:63–69. [PubMed: 15588625]
- 104.
- Jardinaud F, Roques BP, Noble F. Tolerance to the reinforcing effects of morphine in delta9-tetrahydrocannabinol treated mice. Behav. Brain Res. 2006;173:255. [PubMed: 16884789]
- 105.
- Mallet PE, Beninger RJ. Delta9-tetrahydrocannabinol, but not the endogenous cannabinoid receptor ligand anandamide, produces conditioned place avoidance. Life Sci. 1998;62:2431–2439. [PubMed: 9651110]
- 106.
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, La Rana G, Russo R, Calignano A, Gessa GL, Cuomo V, Piomelli D. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. [PubMed: 16541083]
- 107.
- Bardo, et al. 1999
- INTRODUCTION
- RESEARCH DESIGN AND METHODOLOGICAL CONSIDERATIONS
- TRAINING AND TESTING
- DRUG STUDIES USING THE CONDITIONED PLACE PREFERENCE PARADIGM
- THE MESOLIMBIC DOPAMINE SYSTEM IS IMPORTANT FOR CONDITIONED PLACE PREFERENCE
- MECHANISMS MEDIATING CONDITIONED PLACE PREFERENCE FOR COMMON DRUGS OF ABUSE
- CONDITIONED PLACE PREFERENCE VERSUS SELF-ADMINISTRATION
- SUMMARY
- ACKNOWLEDGMENTS
- REFERENCES
- Examining Cocaine Conditioning Place Preference in Mice.[Bio Protoc. 2020]Examining Cocaine Conditioning Place Preference in Mice.Simkevich MJ, Campbell RR, White AO. Bio Protoc. 2020 Apr 20; 10(8):e3595. Epub 2020 Apr 20.
- Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect.[Psychopharmacology (Berl). 2002]Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect.Cunningham CL, Tull LE, Rindal KE, Meyer PJ. Psychopharmacology (Berl). 2002 Apr; 160(4):414-24. Epub 2002 Jan 31.
- Ethanol-induced conditioned place preference, but not aversion, is blocked by treatment with D -penicillamine, an inactivation agent for acetaldehyde.[Psychopharmacology (Berl). 2006]Ethanol-induced conditioned place preference, but not aversion, is blocked by treatment with D -penicillamine, an inactivation agent for acetaldehyde.Font L, Aragon CM, Miquel M. Psychopharmacology (Berl). 2006 Jan; 184(1):56-64. Epub 2005 Dec 13.
- Review [Evaluation of the rewarding effects of drugs by conditioned place preference (CPP) paradigm: properties of volatile organic solvents and uncontrolled newly-abused drugs].[Nihon Arukoru Yakubutsu Igakka...]Review [Evaluation of the rewarding effects of drugs by conditioned place preference (CPP) paradigm: properties of volatile organic solvents and uncontrolled newly-abused drugs].Funada M, Akitake Y, Aoo N. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2008 Oct; 43(5):691-6.
- Review [Usefulness of conditioned place preference (CPP) paradigm and its practical application].[Nihon Yakurigaku Zasshi. 1999]Review [Usefulness of conditioned place preference (CPP) paradigm and its practical application].Suzuki T. Nihon Yakurigaku Zasshi. 1999 Dec; 114(6):365-71.
- Conditioned Place Preference - Methods of Behavior Analysis in NeuroscienceConditioned Place Preference - Methods of Behavior Analysis in Neuroscience
Your browsing activity is empty.
Activity recording is turned off.
See more...
