By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.

Immunobiology: The Immune System in Health and Disease. 5th edition.
Show detailsAlthough the innate immune system lacks the specificity of adaptive immunity, it can distinguish nonself from self. We have already seen, in outline, how this is achieved in the complement system and in the response of macrophages to pathogens. In this part of the chapter we will look more closely at the receptors that activate the innate immune response, both those that recognize pathogens directly and those that signal for a cellular response. Proteins that recognize features common to many pathogens occur as secreted molecules and as receptors on cells of the innate immune system. Their general characteristics are contrasted with the antigen-specific receptors of adaptive immunity in Fig. 2.27. Unlike the receptors that mediate adaptive immunity, the receptors of the innate immune system are typically not clonally distributed; a given set of receptors will be present on all the cells of the same cell type. The binding of pathogens by these receptors gives rise to very rapid responses, which are put into effect without the delay imposed by the clonal expansion of cells needed in the adaptive immune response.

Figure 2.27
The characteristics of receptors of the innate and adaptive immune systems are compared. The innate immune system uses receptors that are encoded by intact genes inherited through the germline, whereas the adaptive immune system uses antigen receptors (more...)
Receptors of the innate immune system mediate a number of different functions. Many are phagocytic receptors that stimulate ingestion of the pathogens they recognize. Some are chemotactic receptors, such as the f-Met-Leu-Phe receptor, which binds the N-formylated peptides produced by bacteria and guides neutrophils to sites of infection. A third function, which may be mediated by some of the phagocytic receptors as well as by specialized signaling receptors, is to induce effector molecules that contribute to the induced responses of innate immunity and molecules that influence the initiation and nature of any subsequent adaptive immune response. In this part of the chapter, we will first examine the recognition properties of the receptors that bind pathogens directly. We will then focus on an evolutionarily primitive recognition and signaling system, originally discovered in the fruit-fly Drosophila melanogaster on account of its role in embryonic development, but now known to play a key role in defense against infection in plants, insects, and vertebrates, including mammals. The receptor mediating these functions in Drosophila is known as Toll. The homologous proteins in mammals have been named the Toll-like receptors and activate phagocytes and tissue dendritic cells to respond to pathogens.
2-15. Receptors with specificity for pathogen surfaces recognize patterns of repeating structural motifs
The surfaces of microorganisms typically bear repeating patterns of molecular structure. The innate immune system recognizes such pathogens by means of receptors that bind features of these regular patterns; these receptors are sometimes known as pattern-recognition molecules. The mannan-binding lectin that initiates the MB-lectin pathway of complement activation (see Section 2-5) is one such receptor, as shown by structural studies of its binding. As illustrated in Fig. 2.28, pathogen recognition and discrimination from self is due to recognition of a particular orientation of certain sugar residues, as well as their spacing, which is found only on pathogenic microbes and not on host cells. Other members of the collectin family also bind pathogens directly and function in innate immunity. As we saw in Section 2-6, the collectin C1q is able to bind directly to pathogen surfaces and initiate complement activation through the classical pathway. In addition, other collectins are made in the liver as part of the acute-phase response, which will be described in the last part of the chapter. The exact structures recognized by these other collectins have not yet been defined, but all collectins have multiple carbohydrate-recognition domains attached to a collagen helix and are thought to bind pathogen surfaces in a similar way to mannan-binding lectin.
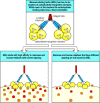
Figure 2.28
Mannan-binding lectin (MBL) binds to patterns of carbohydrate groups in the correct spatial orientation. MBL is a member of the collectin family of proteins, composed of between two to six clusters of carbohydrate-binding lectin domains that interact (more...)
The interaction of these soluble receptors with pathogens leads in turn to binding of the receptor:pathogen complex by phagocytes, either through direct interaction with the pathogen-binding receptor, or through receptors for complement, thus promoting phagocytosis and killing of the bound pathogen (see Section 2-3) and the induction of other cellular responses.
Phagocytes are also equipped with several cell-surface receptors that recognize pathogen surfaces directly. Among these are the macrophage mannose receptor (see Section 2-3). This receptor is a cell-bound C-type lectin that binds certain sugar molecules found on the surface of many bacteria and some viruses, including the human immunodeficiency virus (HIV). Its recognition properties are very similar to those of mannan-binding lectin (see Fig. 2.28) and, like mannan-binding lectin, it is a multipronged molecule with several carbohydrate-recognition domains. Because it is a transmembrane cell-surface receptor, however, it can function directly as a phagocytic receptor. A second set of phagocytic receptors, called scavenger receptors, recognize certain anionic polymers and also acetylated low-density lipoproteins. These receptors are a structurally heterogeneous set of molecules, existing in at least six distinct molecular forms. Some scavenger receptors recognize structures that are shielded by sialic acid on normal host cells. These receptors are involved in the removal of old red blood cells that have lost sialic acid, as well as in the recognition and removal of pathogens. There are other recognition targets, many of which still need to be characterized.
2-16. Receptors on phagocytes can signal the presence of pathogens
In addition to triggering phagocytosis, binding of pathogens by macrophages can also trigger the induced responses of innate immunity, and responses that eventually lead to the induction of adaptive immunity. Not all the receptors on phagocytes appear to transmit such signals. The best-defined activation pathway of this type is triggered through a family of evolutionarily conserved transmembrane receptors that appear to function exclusively as signaling receptors. These receptors, known as the Toll receptors, were first described in the fruit-fly. They appear not to recognize and bind pathogens directly, but clearly are involved in signaling the appropriate response to different classes of pathogen. In the fruit-fly, the Toll receptor itself triggers the production of antifungal peptides in response to fungal infections, while a different member of the Toll family is involved in activating an antibacterial response. In mammals, a Toll-family protein, called Toll-like receptor 4, or TLR-4, signals the presence of LPS by associating with CD14, the macrophage receptor for LPS. TLR-4 is also involved in the immune response to at least one virus, respiratory syncytial virus, although in this case the nature of the stimulating ligand is not known. Another mammalian Toll-like receptor, TLR-2, signals the presence of a different set of microbial constituents, which include the proteoglycans of gram-positive bacteria, although how it recognizes these is not known. TLR-4 and TLR-2 induce similar but distinct signals, as shown by the distinct responses resulting from LPS signaling through TLR-4 and proteoglycan signaling through TLR-2. There are at least nine distinct proteins in this newly discovered family in mammals, and further functions of Toll-like receptors may soon be revealed as mice lacking one or other of these proteins are produced and analyzed.
2-17. The effects of bacterial lipopolysaccharide on macrophages are mediated by CD14 binding to Toll-like receptor 4
Bacterial LPS is a cell-wall component of gram-negative bacteria that has long been known for its ability to induce a dramatic systemic reaction known as septic shock. Perhaps because of this, the best-characterized proteins in innate immunity are the plasma protein LPS-binding protein (LBP), and the receptor protein CD14 (see Section 2-3), which binds LBP-bound LPS. Both LBP and CD14 have leucine-rich repeat motifs. Although the structural details of the binding are not yet characterized, the LPS:LBP complex binds to CD14, which is either free in the plasma or bound to the cell surface by a phosphoinositol glycolipid tail (Fig. 2.29, top two panels). This binding triggers a cell response, but until recently the mechanism by which the signals were transduced across the cell membrane was unknown.
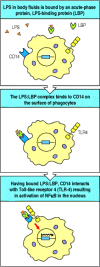
Figure 2.29
Bacterial lipopolysaccharide signals through the Toll-like receptor TLR-4 to activate the transcription factor NFκB. TLR-4 is activated by the binding of bacterial lipopolysaccharide (LPS) through two other proteins. LPS is bound by the soluble LPS-binding (more...)
Mice were discovered that were genetically unresponsive to LPS and did not suffer from septic shock, but had no defects in LBP and CD14. The gene responsible was tracked down by positional cloning and turned out to be the gene for TLR-4, which had suffered inactivating mutations in these mice. The response to LPS could be restored by inserting a transgene encoding TLR-4 into the mouse germline, proving that the defect in TLR-4 was entirely responsible for the loss of responsiveness to LPS. It was subsequently found that TLR-4 binds to the CD14:LBP:LPS complex through a leucine-rich region in CD14’s extracellular domain.
LPS responsiveness is most commonly assessed experimentally by the ability to induce LPS-mediated septic shock. This syndrome is the result of overwhelming secretion of cytokines, particularly of TNF-α, often as a result of an uncontrolled systemic bacterial infection. We will discuss the pathogenesis of septic shock later in this chapter, and will see that it is an undesirable consequence of the same effector actions of TNF-α that are important in containing local infections. The benefits of TLR-4 signaling are clearly illustrated by the mutant mice that lack TLR-4 function; although resistant to septic shock, they are highly sensitive to LPS-bearing pathogens such as Salmonella typhimurium, a natural pathogen of mice. Some people with gram-negative sepsis, an uncontrolled infection of the bloodstream with gram-negative bacteria, which indicates failure to contain an infection locally, have mutations in the TLR-4 gene, showing that TLR-4 is important in protecting against gram-negative sepsis in humans. Only a small fraction of patients have such mutations, however, so there may be other specific defects that contribute to a failure to respond adequately to gram-negative bacteria.
When TLR-4 binds to CD14 complexed with its LBP:LPS ligand, TLR-4 sends a signal to the nucleus that activates the transcription factor NFκB (Fig. 2.29, bottom panel). We will describe the signaling pathway used by TLR-4 in detail in Chapter 6. This signaling pathway was first discovered as the pathway used by the Toll receptor to determine dorsoventral body pattern during embryogenesis in the fruit-fly Drosophila, and we will therefore call it the Toll pathway. The pathway was recently shown to participate in defense against infection in adult flies, and a similar pathway is used by plants in their defense against viruses. Thus the Toll pathway is an ancient signaling pathway that appears to be used in innate immune defense in most or all multicellular organisms.
2-18. Activation of Toll-like receptors triggers the production of pro-inflammatory cytokines and chemokines, and the expression of co-stimulatory molecules
The Toll signaling pathway in adult flies induces the production of several antimicrobial peptides that contribute to the fly’s defense against infection. In humans and all other vertebrates examined, activation of NFκB by the Toll pathway leads to the production of several important mediators of innate immunity, such as cytokines and chemokines. At the same time, Toll signaling induces molecules that are essential for the induction of adaptive immune responses. These are known as co-stimulatory molecules, and they will be considered in detail in Chapter 8. The co-stimulatory molecules, called B7.1 (CD80) and B7.2 (CD86), are cell-surface proteins that are expressed by both macrophages and tissue dendritic cells in response to LPS signaling through TLR-4. It is the presence of these molecules along with the microbial antigens presented by macrophages and dendritic cells (see Section 1-6) that activates the CD4 T cells required to initiate most adaptive immune responses. To encounter a CD4 T cell, the antigen-presenting dendritic cell must migrate to a nearby lymph node through which circulating T cells pass, and this migration is stimulated by cytokines such as TNF-α, which are also induced through signaling by TLR-4. Thus, the activation of adaptive immunity depends on molecules induced as a consequence of innate immune recognition and signaling (Fig. 2.30).
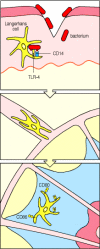
Figure 2.30
Bacterial LPS induces changes in Langerhans’ cells, stimulating them to migrate and initiate adaptive immunity to infection by activating CD4 T cells. Langerhans’ cells are immature dendritic cells resident in the skin. In the case of (more...)
Substances such as LPS that induce co-stimulatory activity have been used for years in mixtures that are co-injected with protein antigens to enhance their immunogenicity. These substances are known as adjuvants (see Appendix I, Section A-4), and it was found empirically that the best adjuvants contained microbial components. Pathogen components that can induce macrophages and tissue dendritic cells to express co-stimulatory molecules and cytokines include glycans, mannans, and the mycobacterial extract muramyl dipeptide. The receptor TLR-2 has recently been shown to be essential for the response to proteoglycans and it is thought that all these pathogen components are recognized by pattern-recognition molecules that then signal, often through Toll-like receptors, to induce co-stimulatory molecules and cytokines. The exact profile of cytokines produced varies according to the receptors involved and, as we will see in Chapters 8 and 10, this will in turn influence the functional character of the adaptive immune response that develops in their presence. In this way, the ability of the innate immune system to discriminate between different types of pathogen is used to ensure an appropriate type of adaptive immune response.
Summary
The innate immune system uses a diversity of receptors to recognize and respond to pathogens. Those that recognize pathogen surfaces directly often bind to repeating patterns, for example, of carbohydrate or lipid moieties, that are characteristic of microbial surfaces but are not found on host cells. Some of these receptors, for example, the macrophage mannose receptor, directly stimulate phagocytosis, while others are produced as secreted molecules that promote the phagocytosis of pathogens by opsonization or by the activation of complement. The innate immune system receptors that recognize pathogens also have an important role in signaling for the induced responses responsible for local inflammation, the recruitment of new effector cells, the containment of local infection, and the initiation of an adaptive immune response. Such signals can be transmitted through a family of signaling receptors, known as the Toll-like receptors, that have been highly conserved across species and that serve to activate host defense through a signaling pathway that operates in most multicellular organisms. In mammals, Toll-like receptors also play a key role in enabling the initiation of adaptive immunity. TLR-4 detects the presence of gram-negative bacteria through its association with the peripheral membrane protein CD14, which is a receptor for bacterial LPS bound to its binding protein, LBP. TLR-2 signals in response to microbial proteoglycans, although how it recognizes them is not yet known. Ligation of TLR-2 or TLR-4 activates an evolutionarily ancient signaling pathway that leads to the activation of the transcription factor NFκB, and the induction of a variety of genes, including genes for cytokines, chemokines, and co-stimulatory molecules that play essential roles in directing the course of the adaptive immune response later in infection.
- Receptors with specificity for pathogen surfaces recognize patterns of repeating structural motifs
- Receptors on phagocytes can signal the presence of pathogens
- The effects of bacterial lipopolysaccharide on macrophages are mediated by CD14 binding to Toll-like receptor 4
- Activation of Toll-like receptors triggers the production of pro-inflammatory cytokines and chemokines, and the expression of co-stimulatory molecules
- Summary
- Receptors of the innate immune system - ImmunobiologyReceptors of the innate immune system - Immunobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...