By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002.

Molecular Biology of the Cell. 4th edition.
Show detailsIt is through another class of specializations of the epithelium covering our body surface that we sense the smells, sounds and sights of the external world. The sensory tissues of the nose, the ears, and the eyes—and, indeed, if we look back to origins in the early embryo, the whole of the central nervous system—all arise as parts of the same sheet of cells, the ectoderm, that gives rise to the epidermis (see Chapter 21). These structures have several basic features in common, and their development is governed by related systems of genes (discussed in Chapter 21). They all retain an epithelial organization, but it is very different from that of the ordinary epidermis or of the glands that derive from it.
The nose, the ear, and the eye are complex organs, with elaborate devices to collect signals from the external world and to deliver them, filtered and concentrated, to the sensory epithelia, where they can trigger effects on the nervous system. The sensory epithelium in each organ is the key component, although it is small relative to all the ancillary apparatus. It is the part that has been most highly conserved in evolution, not only from one vertebrate to another, but also between vertebrates and invertebrates.
Within each sensory epithelium lie sensory cells that act as transducers, converting signals from the outside world into an electrical form that can be interpreted by the nervous system. In the nose, the sensory transducers are olfactory sensory neurons; in the ear, auditory hair cells; and in the eye, photoreceptors. All of these cell types are either neurons or neuron-like. Each carries at its apical end a specialized structure that detects the external stimulus and converts it to a change in the membrane potential. At its basal end, each makes synapses with neurons that relay the sensory information to specific sites in the brain.
Olfactory Sensory Neurons Are Continually Replaced
In the olfactory epithelium of the nose (Figure 22-10A), a subset of the epithelial cells differentiate as olfactory sensory neurons. These cells have modified, immotile cilia on their free surfaces (see Figure 15-43), containing odorant receptor proteins, and a single axon extending from their basal end towards the brain (Figure 22-10B). The neurons are held in place and separated from one another by supporting cells that span the thickened epithelium and have properties similar to those of glial cells in the central nervous system. The sensory surfaces are kept moist and protected by a layer of fluid secreted by cells sequestered in glands that communicate with the exposed surface. Even with this protection, however, each olfactory neuron survives for only a month or two, and so a third class of cells—the basal cells—is present in the epithelium to generate replacements for the olfactory neurons that are lost. The basal cells are stem cells for the production of the neurons.

Figure 22-10
Olfactory epithelium and olfactory neurons. (A) Olfactory epithelium consists of supporting cells, basal cells, and olfactory sensory neurons. The basal cells are the stem cells for production of the olfactory neurons. Six to eight modified cilia project (more...)
As discussed in Chapter 15, each olfactory neuron expresses most probably only one of the hundreds of odorant receptor genes in the genome. This determines which odorants the cell responds to. But regardless of the odor, the response of every olfactory neuron is the same—a train of action potentials sent back along its axon to the brain. The discriminating sensibility of an individual olfactory neuron is therefore useful only if its axon delivers its messages to the specific relay station in the brain that is dedicated to the particular range of odors that the neuron senses. These relay stations are called glomeruli. They are located in structures called the olfactory bulbs (one on each side of the brain), with about 1800 glomeruli in each bulb (in the mouse). Olfactory neurons expressing the same odorant receptor are widely scattered in the olfactory epithelium, but their axons all converge on the same glomerulus (Figure 22-10C). As new olfactory neurons are generated, replacing those that die, they must in turn send their axons to the correct glomerulus. The odorant receptor proteins thus have been proposed to have a second function: helping to guide the growing tips of the new axons along specific paths to the appropriate target glomeruli in the olfactory bulbs. If it were not for the continual operation of this guidance system, a rose might smell in one month like a lemon, in the next like rotting fish.
Auditory Hair Cells Have to Last a Lifetime
The sensory epithelium responsible for hearing is the most precisely and minutely engineered of all the tissues in the body (Figure 22-11). Its sensory cells, the auditory hair cells, are held in a rigid framework of supporting cells and overlaid by a mass of extracellular matrix (the tectorial membrane), in a structure called the organ of Corti. The hair cells convert mechanical stimuli into electrical signals. Each has a characteristic organ-pipe array of giant microvilli (called stereocilia) protruding from its surface as rigid rods, filled with cross-linked actin filaments, and arranged in ranks of graded height. The dimensions of each such array are specified with extraordinary accuracy according to the location of the hair cell in the ear and the frequency of sound that it has to respond to. Sound vibrations rock the organ of Corti, causing the bundles of stereocilia to tilt (Figure 22-12) and mechanically gated ion channels in the membranes of the stereocilia to open or close (Figure 22-13). The flow of electric charge carried into the cell by the ions alters the membrane potential and thereby controls the release of neurotransmitter at the cell's basal end, where the cell synapses with a nerve ending.
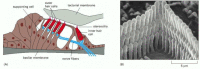
Figure 22-11
Auditory hair cells. (A) A diagrammatic cross section of the auditory apparatus (the organ of Corti) in the inner ear of a mammal shows the auditory hair cells held in an elaborate epithelial structure of supporting cells and overlaid by a mass of extracellular (more...)
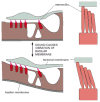
Figure 22-12
How a relative movement of the overlying extracellular matrix (the tectorial membrane) tilts the stereocilia of auditory hair cells in the organ of Corti in the inner ear of a mammal. The stereocilia behave as rigid rods hinged at the base and bundled (more...)
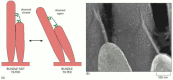
Figure 22-13
How a sensory hair cell works. (A) The cell functions as a transducer, generating an electrical signal in response to sound vibrations that rock the organ of Corti and so cause the stereocilia to tilt. A fine filament runs more or less vertically upward (more...)
In humans and other mammals, the auditory hair cells, unlike olfactory neurons, have to last a lifetime. If they are destroyed by disease, toxins, or excessively loud noise, they are not regenerated and the resultant hearing loss is permanent. But in other vertebrates, when auditory hair cells are destroyed, the supporting cells are triggered to divide and behave as stem cells, generating progeny that can differentiate as replacements for the hair cells that are lost. With better understanding of how this regeneration process is regulated, we may one day be able to induce the auditory epithelium to repair itself in humans also.
Most Permanent Cells Renew Their Parts: the Photoreceptor Cells of the Retina
The neural retina is the most complex of the sensory epithelia. It consists of several cell layers organized in a way that seems perverse. The neurons that transmit signals from the eye to the brain (called retinal ganglion cells) lie closest to the external world, so that the light, focused by the lens, must pass through them to reach the photoreceptor cells. The photoreceptors, which are classified as rods or cones, according to their shape, lie with their photoreceptive ends, or outer segments, partly buried in the pigment epithelium (Figure 22-14). Rods and cones contain different rhodopsins—photosensitive complexes of opsin protein with the visual pigment retinal. Rods are especially sensitive at low light levels, while cones (of which there are three types, each with a different opsin, giving a different spectral response) detect color and fine detail.
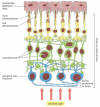
Figure 22-14
The structure of the retina. The stimulation of the photoreceptors by light is relayed via interneurons to the ganglion cells, which convey the signal to the brain. The spaces between neurons and between photoreceptors in the neural retina are occupied (more...)
The outer segment of a photoreceptor appears to be a modified cilium with a characteristic ciliumlike arrangement of microtubules in the region where the outer segment is connected to the rest of the cell (Figure 22-15). The remainder of the outer segment is almost entirely filled with a dense stack of membranes in which the photosensitive complexes are embedded; light absorbed here produces an electrical response, as discussed in Chapter 15. At their opposite ends, the photoreceptors form synapses on interneurons, which relay the signal to the retinal ganglion cells (see Figure 22-14).
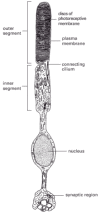
Figure 22-15
A rod photoreceptor.
Photoreceptors in humans, like human auditory hair cells, are permanent cells that do not divide and are not replaced if destroyed by disease or by a mis-directed laser beam. But the photosensitive rhodopsin molecules are not permanent but are continually degraded and replaced. In rods (although not, curiously, in cones), this turnover is organized in an orderly production line, which can be analyzed by following the passage of a cohort of radiolabeled protein molecules through the cell after a short pulse of radioactive amino acid (Figure 22-16). The radiolabeled proteins can be followed from the Golgi apparatus in the inner segment of the cell to the base of the stack of membranes in the outer segment. From here they are gradually displaced toward the tip as new material is fed into the base of the stack. Finally (after about 10 days in the rat), on reaching the tip of the outer segment, the labeled proteins and the layers of membrane in which they are embedded are phagocytosed (chewed off and digested) by the cells of the pigment epithelium.
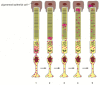
Figure 22-16
Turnover of membrane protein in a rod cell. Following a pulse of 3H-leucine, the passage of radiolabeled proteins through the cell is followed by autoradiography. Red dots indicate sites of radioactivity. The method reveals only the 3H-leucine that has (more...)
This example illustrates a general point: even though individual cells of certain types persist, very little of the adult body consists of the same molecules that were laid down in the embryo.
Summary
Most sensory receptor cells, like epidermal cells and nerve cells, derive from the epithelium forming the outer surface of the embryo. They transduce external stimuli into electrical signals, which they relay to neurons via chemical synapses. Olfactory receptor cells in the nose are themselves full-fledged neurons, sending their axons to the brain. They have a lifetime of only a month or two, and are continually replaced by new cells derived from stem cells in the olfactory epithelium. Each olfactory neuron expresses just one of the hundreds of different olfactory receptor proteins for which genes exist in the genome, and the axons from all olfactory neurons expressing the same receptor protein navigate to the same glomeruli in the olfactory bulbs of the brain.
Auditory hair cells—the receptor cells for sound—unlike olfactory receptor cells, have to last a lifetime, in mammals at least. They have no axon but make synaptic contact with nerve terminals in the auditory epithelium. They take their name from the hair-like bundle of stereocilia (giant microvilli) on their outer surface. Sound vibrations tilt the bundle, pulling mechanically gated ion channels on the stereocilia into an open configuration to excite the cell electrically.
Photoreceptor cells in the retina of the eye absorb photons in rhodopsin molecules held in stacks of membrane in the photoreceptor outer segments, triggering an electrical excitation by a more indirect intracellular signaling pathway. Although the photoreceptor cells themselves are permanent and irreplaceable, the stacks of rhodopsin-rich membrane that they contain undergo continual renewal.
- Sensory Epithelia - Molecular Biology of the CellSensory Epithelia - Molecular Biology of the Cell
Your browsing activity is empty.
Activity recording is turned off.
See more...